



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
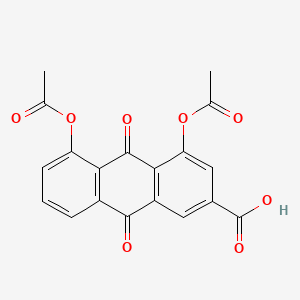

1. 1,8-diacetoxy-3-carboxyanthraquinone
2. 2-anthracenecarboxylic Acid, 4,5-bis(acetyloxy)-9,10-dihydro-9,10-dioxo-
3. 4,5-bis(acetyloxy)-9,10-dihydro-9,10-dioxo-2-anthracenecarboxylic Acid
4. 4,5-diacetoxyanthraquinone-2-carboxylic Acid
5. 9,10-dihydro-4,5-dihydroxy-9,10-dioxo-2-anthroic Acid, Diacetate
6. Ac-201
7. Ac-203
8. Artrodar
9. Diacerhein
10. Diacetyl-rhein
11. Diacetylrhein
12. Diora
13. Fisiodar
14. M-01ax21
15. M01ax21
16. Rhein Diacetate
17. Sf-277
18. Sf277
19. Verboril
20. Zondar
1. 13739-02-1
2. Diacerhein
3. Diacetylrhein
4. Artrodar
5. Fisiodar
6. 1,8-diacetoxy-3-carboxyanthraquinone
7. Diacerin
8. Sf-277
9. Verboril
10. Zondar
11. Diora
12. 4,5-diacetyloxy-9,10-dioxoanthracene-2-carboxylic Acid
13. M01ax21
14. Ac-201
15. 2-anthracenecarboxylic Acid, 4,5-bis(acetyloxy)-9,10-dihydro-9,10-dioxo-
16. 4,5-bis(acetyloxy)-9,10-dihydro-9,10-dioxo-2-anthracenecarboxylic Acid
17. 4,5-diacetoxyanthraquinone-2-carboxylic Acid
18. M-01ax21
19. Diacerhein; Diacetylrhein
20. Mfcd00468030
21. Nsc-758147
22. Mls000028577
23. 4hu6j11el5
24. 4,5-diacetoxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic Acid
25. 9,10-dihydro-4,5-dihydroxy-9,10-dioxo-2-anthroic Acid, Diacetate
26. 4,5-diacetylrhein
27. Ncgc00018274-04
28. Smr000058958
29. 4,5-bis(acetyloxy)-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic Acid
30. Dsstox_cid_25636
31. Dsstox_rid_81017
32. Diacerein [inn]
33. Dsstox_gsid_45636
34. Diacereine [french]
35. Diacereinum [latin]
36. Diacereina [spanish]
37. 4,5-diacetoxy-9,10-diketo-anthracene-2-carboxylic Acid
38. 4,5-diacetyloxy-9,10-dioxo-2-anthracenecarboxylic Acid
39. Diacereina
40. Diacereine
41. Diacereinum
42. Rhein Diacetate
43. Rhein, Diacetate
44. Cas-13739-02-1
45. Sr-01000003156
46. Einecs 237-310-2
47. Brn 2184909
48. Unii-4hu6j11el5
49. Gesamtmatrix
50. Art 50
51. 1,8-diacetoxyanthraquinone-3-carboxylic Acid
52. Diacerein- Bio-x
53. Zondar (tn)
54. Spectrum_001876
55. Specplus_000643
56. 4,5-diacetoxy-9,10-dihydro-9,10-dioxo-2-anthrylcarbonsaeure
57. 9,10-dihydro-4,5-diacetoxy-9,10-2-anthracenecarboxylic Acid
58. Diacerein [mi]
59. Diacerein (usan/inn)
60. Diacerein [usan:inn]
61. Diacerein [usan]
62. 9,10-dihydro-4,5-dihydroxy-9,10-dioxo-2-anthroic Acid Diacetate
63. Spectrum2_000823
64. Spectrum3_000937
65. Spectrum4_001036
66. Spectrum5_001819
67. Diacerein Impurity Mixture
68. Diacerein [mart.]
69. Diacerein [who-dd]
70. Schembl25784
71. Kbiogr_001591
72. Kbioss_002400
73. Rhein Diacetate [mi]
74. 3-10-00-04790 (beilstein Handbook Reference)
75. Cid_26248
76. Chembl41286
77. Divk1c_006739
78. Spectrum1502010
79. Spbio_000745
80. 4,5-diacetoxy-9,10-dioxo-anthracene-2-carboxylic Acid
81. Diacerein [ep Monograph]
82. Diacerein, >=95% (hplc)
83. Dtxsid4045636
84. Bdbm32018
85. Chebi:94708
86. Gtpl10800
87. Kbio1_001683
88. Kbio2_002395
89. Kbio2_004963
90. Kbio2_007531
91. Kbio3_001974
92. Hms3652d06
93. Hms3714b20
94. Pharmakon1600-01502010
95. Bcp10834
96. Hy-n0283
97. Who 5371
98. Zinc3812842
99. Tox21_110856
100. Bbl011075
101. Ccg-40287
102. Nsc758147
103. S4267
104. Stk802271
105. 2-anthroic Acid, 9,10-dihydro-4,5-dihydroxy-9,10-dioxo-, Diacetate
106. Akos005622705
107. Tox21_110856_1
108. Db11994
109. Ks-5088
110. Kw-4800
111. Nsc 758147
112. Ncgc00018274-01
113. Ncgc00018274-02
114. Ncgc00018274-03
115. Ncgc00018274-05
116. Ncgc00022114-03
117. Am807992
118. Bd164367
119. Sbi-0052833.p002
120. B1726
121. D4061
122. Ft-0603096
123. Sw199355-2
124. D07270
125. Ab00053327_14
126. 739d021
127. A807252
128. Q413178
129. Sr-01000003156-2
130. Sr-01000003156-3
131. Sr-01000003156-4
132. W-108237
133. Diacerein, European Pharmacopoeia (ep) Reference Standard
134. 4,5-diacetoxy-9,10-dihydro-9,10-dioxoanthracene-2-carboxylic Acid
135. 4,5-diacetyloxy-9,10-bis(oxidanylidene)anthracene-2-carboxylic Acid
136. 4,5-bis(acetoxy)-9,10-dihydro-9,10-dioxo-2-anthracenecarboxylic Acid
137. 112118-18-0
| Molecular Weight | 368.3 g/mol |
|---|---|
| Molecular Formula | C19H12O8 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 368.05321734 g/mol |
| Monoisotopic Mass | 368.05321734 g/mol |
| Topological Polar Surface Area | 124 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 683 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of osteoarthritis affecting the hip or knee.
Decreases inflammation and cartilage destruction and also corrects altered osteoblast acitivity.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AX - Other antiinflammatory and antirheumatic agents, non-steroids
M01AX21 - Diacerein
Absorption
Bioavailability of 50-60%. Entirely converted to the active metabolite rhein [DB13174] before reaching systemic circulation.
Route of Elimination
37% excreted in urine and 53% in feces as estimated in rats.
Volume of Distribution
15-60L.
Clearance
Total CL is 1.5L/h and renal CL is 0.1L/h.
Entirely converted to rhien [DB13174] through double deacetylation before reaching systemic circulation. Rhein [DB13174] is further metabolized to rhein glucuronide and rhein sulfate.
4-10h.
Diacerein's active metabolite rhein [DB13174] reduces cartilage destruction by decreasing expression of matrix metalloproteinase (MMP)-1 and -3 as well as upregulating tissue inhibitor of matrix metalloproteinases which serve to reduce the activity of several MMPs. The anti-inflammatory action of rhein reduces the level of interleukin-1beta activity which plays a large role in reduction of extracellular matrix production, MMP activity, and continued inflammation. Rhein reduces abnormal osteoblast synthetic activity through an unknown mechanism.


