
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
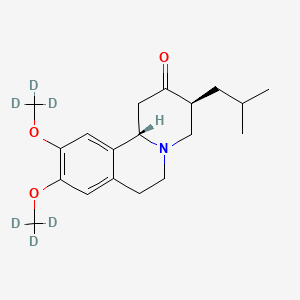

1. Deutetrabenazine
1. Deutetrabenazine
2. Dutetrabenazine
3. P341g6w9nb
4. Sd-809
5. (3rs,11brs)-9,10-di((2h3)methoxy)-3-(2-methylpropyl)-1,3,4,6,7,11b-hexahydro-2h-benzo(a)quinolizin-2-one
6. 2h-benzo(a)quinolizin-2-one, 1,3,4,6,7,11b-hexahydro-9,10-di(methoxy-d3)-3-(2-methylpropyl)-, (3r,11br)-rel-
7. Sd809
8. Unii-p341g6w9nb
9. Deutetrabenazine [usan:inn]
10. Sd 809
11. Deutetrabenazine [usan]
12. Deutetrabenazine [inn]
13. Schembl16228022
14. Hsdb 8414
15. Deutetrabenazine [who-dd]
16. Deutetrabenazine [orange Book]
17. At21717
18. Db12161
| Molecular Weight | 323.5 g/mol |
|---|---|
| Molecular Formula | C19H27NO3 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 323.23675420 g/mol |
| Monoisotopic Mass | 323.23675420 g/mol |
| Topological Polar Surface Area | 38.8 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 425 |
| Isotope Atom Count | 6 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | AUSTEDO |
| Active Ingredient | DEUTETRABENAZINE |
| Company | TEVA BRANDED PHARM (Application Number: N208082. Patents: 8524733, 9233959, 9296739, 9550780, 9814708) |
Adrenergic Uptake Inhibitors
National Library of Medicine's Medical Subject Headings. Deutetrabenazine. Online file (MeSH, 2018). Available from, as of April 5, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Dutetrabenazine is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of April 5, 2018: https://clinicaltrials.gov/
Austedo is indicated for the treatment of chorea associated with Huntington's disease. /Included in US product label/
NIH; DailyMed. Current Medication Information for Austedo (Deutetrabenazine) Tablet, Coated (Updated: September 11, 2017). Available from, as of April 5, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7ea3c60a-45c7-44cc-afc2-d87fa53993c0
Austedo is indicated for the treatment of tardive dyskinesia in adults. /Included in US product label/
NIH; DailyMed. Current Medication Information for Austedo (Deutetrabenazine) Tablet, Coated (Updated: September 11, 2017). Available from, as of April 5, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7ea3c60a-45c7-44cc-afc2-d87fa53993c0
/EXPL THER/ Deutetrabenazine, an inhibitor of vesicular monoamine transporter type 2 (VMAT2) depletes presynaptic dopamine and is useful in the treatment of hyperkinetic movement disorders. This study explored the safety, tolerability, and preliminary efficacy of deutetrabenazine in adolescents with moderate-to-severe tics associated with Tourette syndrome (TS). In this open-label study of 12-18-year-old patients with TS-related tics, deutetrabenazine was titrated up to 36 mg/day over 6 weeks to adequately suppress tics without bothersome adverse effects (AEs), followed by maintenance at optimal dose for 2 weeks. An independent blinded rater assessed tic severity using the Yale Global Tic Severity Scale (YGTSS), which was the primary outcome measure. Secondary outcome measures included the TS Clinical Global Impression (TS-CGI) and TS Patient Global Impression of Change (TS-PGIC). Twenty-three enrolled patients received deutetrabenazine and had at least 1 post-baseline YGTSS assessment. The mean (SD [standard deviation]) baseline YGTSS Total Tic Severity Score (TTS) was 31.6 (7.9) and had decreased by 11.6 (8.2) points at week 8, a 37.6% reduction in tic severity (p<0.0001). The TS-CGI score improved by 1.2 (0.81) points (p<0.0001) and the TS-PGIC results at week 8 indicated that 76% of patients were much improved or very much improved compared with baseline. The mean (SD) daily deutetrabenazine dose at week 8 was 32.1 (6.6) mg (range 18-36 mg). One week after withdrawal of deutetrabenazine, the TTS scores increased by 5.6 (8.4) points, providing confirmation of the drug effect. No serious or severe adverse events were reported. The results of this open-label 8-week study suggest that deutetrabenazine is safe and associated with improvement in tic severity in adolescents with TS and troublesome tics.
PMID:27917309 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5133390 Jankovic J et al; Tremor Other Hyperkinet Mov (N Y). 6: 422 (2016)
/BOXED WARNING/ WARNING: DEPRESSION AND SUICIDALITY IN PATIENTS WITH HUNTINGTON'S DISEASE. Austedo can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington's disease. Anyone considering the use of Austedo must balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Patients, their caregivers, and families should be informed of the risk of depression and suicidality and should be instructed to report behaviors of concern promptly to the treating physician. Particular caution should be exercised in treating patients with a history of depression or prior suicide attempts or ideation, which are increased in frequency in Huntington's disease. Austedo is contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
NIH; DailyMed. Current Medication Information for Austedo (Deutetrabenazine) Tablet, Coated (Updated: September 11, 2017). Available from, as of April 5, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7ea3c60a-45c7-44cc-afc2-d87fa53993c0
Huntington's disease is a progressive disorder characterized by changes in mood, cognition, chorea, rigidity, and functional capacity over time. Vesicular monoamine transporter 2 (VMAT2) inhibitors, including deutetrabenazine, may cause a worsening in mood, cognition, rigidity, and functional capacity. Prescribers should periodically reevaluate the need for deutetrabenazine in their patients by assessing the effect on chorea and possible adverse effects, including sedation/somnolence, depression and suicidality, parkinsonism, akathisia, restlessness, and cognitive decline. It may be difficult to distinguish between adverse reactions and progression of the underlying disease; decreasing the dose or stopping the drug may help the clinician to distinguish between the two possibilities. In some patients, the underlying chorea itself may improve over time, decreasing the need for deutetrabenazine.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017
Deutetrabenazine may increase the risk of akathisia, agitation, and restlessness in patients with Huntington's disease and tardive dyskinesia. In a 12-week, double-blind, placebo-controlled trial in Huntington's disease patients, akathisia, agitation, or restlessness was reported by 4% of patients treated with deutetrabenazine, compared to 2% of patients on placebo; in patients with tardive dyskinesia, 2% of patients treated with deutetrabenazine and 1% of patients on placebo experienced these events. Patients receiving deutetrabenazine should be monitored for signs and symptoms of restlessness and agitation, as these may be indicators of developing akathisia. If a patient develops akathisia during treatment with deutetrabenazine, the deutetrabenazine dose should be reduced; some patients may require discontinuation of therapy.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017
A potentially fatal symptom complex sometimes referred to as neuroleptic malignant syndrome (NMS) has been reported in association with drugs that reduce dopaminergic transmission. While NMS has not been observed in patients receiving deutetrabenazine, it has been observed in patients receiving tetrabenazine (a closely related VMAT2 inhibitor). Clinicians should be alerted to the signs and symptoms associated with NMS. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatinine phosphokinase, myoglobinuria, rhabdomyolysis, and acute renal failure. The diagnosis of NMS can be complicated; other serious medical illness (e.g., pneumonia, systemic infection) and untreated or inadequately treated extrapyramidal disorders can present with similar signs and symptoms. Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system pathology. /Tetrabenazine/
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017
For more Drug Warnings (Complete) data for Deutetrabenazine (14 total), please visit the HSDB record page.
Indicated for the treatment of chorea associated with Huntingtons disease.
FDA Label
In clinical trials, there was an evidence of clinical effectiveness of deutetrabenazine in improving the symptoms of involuntary movements in patient with tardive dyskinesia by reducing the mean Abnormal Involuntary Movement Scale (AIMS) score. In a randomized, double-blind, placebo-controlled crossover study in healthy male and female subjects, single dose administration of 24 mg deutetrabenazine results in an approximately 4.5 msec mean increase in QTc. Effects at higher exposures to deutetrabenazine or its metabolites have not been evaluated. Deutetrabenazine and its metabolites were shown to bind to melanin-containing tissues including eyes, skin and fur in pigmented rats. After a single oral dose of radiolabeled deutetrabenazine, radioactivity was still detected in eye and fur at 35 days following dosing.
N - Nervous system
N07 - Other nervous system drugs
N07X - Other nervous system drugs
N07XX - Other nervous system drugs
N07XX16 - Deutetrabenazine
Absorption
The extent of absorption is 80% with oral deutetrabenazine. As deutetrabenazine is extensively metabolized to its main active metabolites following administration, linear dose dependence of peak plasma concentrations (Cmax) and AUC was observed for the metabolites after single or multiple doses of deutetrabenazine (6 mg to 24 mg and 7.5 mg twice daily to 22.5 mg twice daily). Cmax of deuterated -HTBZ and -HTBZ are reached within 3-4 hours post-dosing. Food may increase the Cmax of -HTBZ or -HTBZ by approximately 50%, but is unlikely to have an effect on the AUC.
Route of Elimination
Deutetrabenazine is mainly excreted in the urine as metabolites. In healthy subjects, about 75% to 86% of the deutetrabenazine dose was excreted in the urine, and fecal recovery accounted for 8% to 11% of the dose. Sulfate and glucuronide conjugates of the -HTBZ and -HTBZ, as well as products of oxidative metabolism, accounted for the majority of metabolites in the urine. -HTBZ and -HTBZ metabolites accounted for less than 10% of the administered dose in the urine.
Volume of Distribution
The median volume of distribution (Vc/F) of the -HTBZ, and the -HTBZ metabolites of deutetrabenazine are approximately 500 L and 730 L, respectively. Human PET-scans of tetrabenazine indicate rapid distribution to the brain, with the highest binding in the striatum and lowest binding in the cortex. Similar distribution pattern is expected for deutetrabenazine.
Clearance
In patients with Huntington's disease, the median clearance values (CL/F) of the -HTBZ, and the -HTBZ metabolites of deutetrabenazine are approximately 47 L/hour and 70 L/hour, respectively.
Results of PET-scan studies in humans show that following intravenous injection of (11)C-labeled tetrabenazine or alpha-dihydrotetrabenazine, radioactivity is rapidly distributed to the brain, with the highest binding in the striatum and lowest binding in the cortex.
NIH; DailyMed. Current Medication Information for Austedo (Deutetrabenazine) Tablet, Coated (Updated: September 11, 2017). Available from, as of April 5, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7ea3c60a-45c7-44cc-afc2-d87fa53993c0
Following oral administration of deutetrabenazine, the extent of absorption is at least 80%.
NIH; DailyMed. Current Medication Information for Austedo (Deutetrabenazine) Tablet, Coated (Updated: September 11, 2017). Available from, as of April 5, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7ea3c60a-45c7-44cc-afc2-d87fa53993c0
In a mass balance study in 6 healthy subjects, 75% to 86% of the deutetrabenazine dose was excreted in the urine, and fecal recovery accounted for 8% to 11% of the dose. Urinary excretion of the alpha-dihydrotetrabenazine and beta-dihydrotetrabenazine metabolites from deutetrabenazine each accounted for less than 10% of the administered dose. Sulfate and glucuronide conjugates of the alpha-dihydrotetrabenazine and beta-dihydrotetrabenazine metabolites of deutetrabenazine, as well as products of oxidative metabolism, accounted for the majority of metabolites in the urine.
NIH; DailyMed. Current Medication Information for Austedo (Deutetrabenazine) Tablet, Coated (Updated: September 11, 2017). Available from, as of April 5, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7ea3c60a-45c7-44cc-afc2-d87fa53993c0
Austedo is primarily renally eliminated in the form of metabolites.
NIH; DailyMed. Current Medication Information for Austedo (Deutetrabenazine) Tablet, Coated (Updated: September 11, 2017). Available from, as of April 5, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7ea3c60a-45c7-44cc-afc2-d87fa53993c0
Deutetrabenazine undergoes extensive hepatic biotransformation mediated by carbonyl reductase to form its major active metabolites, -HTBZ and -HTBZ. These metabolites may subsequently metabolized to form several minor metabolites, with major contribution of CYP2D6 and minor contributions of CYP1A2 and CYP3A4/5.
In a mass balance study in 6 healthy subjects, 75% to 86% of the deutetrabenazine dose was excreted in the urine, and fecal recovery accounted for 8% to 11% of the dose. Urinary excretion of the alpha-dihydrotetrabenazine and beta-dihydrotetrabenazine metabolites from deutetrabenazine each accounted for less than 10% of the administered dose. Sulfate and glucuronide conjugates of the alpha-dihydrotetrabenazine and beta-dihydrotetrabenazine metabolites of deutetrabenazine, as well as products of oxidative metabolism, accounted for the majority of metabolites in the urine.
NIH; DailyMed. Current Medication Information for Austedo (Deutetrabenazine) Tablet, Coated (Updated: September 11, 2017). Available from, as of April 5, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7ea3c60a-45c7-44cc-afc2-d87fa53993c0
In vitro experiments in human liver microsomes demonstrate that deutetrabenazine is extensively biotransformed, mainly by carbonyl reductase, to its major active metabolites, alpha-dihydrotetrabenazine and beta-dihydrotetrabenazine, which are subsequently metabolized primarily by CYP2D6, with minor contributions of CYP1A2 and CYP3A4/5, to form several minor metabolites.
NIH; DailyMed. Current Medication Information for Austedo (Deutetrabenazine) Tablet, Coated (Updated: September 11, 2017). Available from, as of April 5, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7ea3c60a-45c7-44cc-afc2-d87fa53993c0
The half-life of total (+)-HTBZ from deutetrabenazine is approximately 9 to 10 hours.
The half-life of total (alpha+beta)-dihydrotetrabenazine from deutetrabenazine is approximately 9 to 10 hours.
NIH; DailyMed. Current Medication Information for Austedo (Deutetrabenazine) Tablet, Coated (Updated: September 11, 2017). Available from, as of April 5, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=7ea3c60a-45c7-44cc-afc2-d87fa53993c0
The precise mechanism of action of deutetrabenazine in mediating its anti-chorea effects is not fully elucidated. Deutetrabenazine reversibly depletes the levels of monoamines, such as dopamine, serotonin, norepinephrine, and histamine, from nerve terminals via its active metabolites. The major circulating metabolites are -dihydrotetrabenazine [HTBZ] and -HTBZ that act as reversible inhibitors of VMAT2. Inhibition of VMAT2 results in decreased uptake of monoamines into synaptic terminal and depletion of monoamine stores from nerve terminals. Deutetrabenazine contains the molecule deuterium, which is a naturally-occurring, nontoxic hydrogen isotope but with an increased mass relative to hydrogen. Placed at key positions, deuterium forms a stronger hydrogen bond with carbon that requires more energy for cleavage, thus attenuating CYP2D6-mediated metabolism without having any effect on the therapeutic target.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
60
PharmaCompass offers a list of Deutetrabenazine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Deutetrabenazine manufacturer or Deutetrabenazine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Deutetrabenazine manufacturer or Deutetrabenazine supplier.
PharmaCompass also assists you with knowing the Deutetrabenazine API Price utilized in the formulation of products. Deutetrabenazine API Price is not always fixed or binding as the Deutetrabenazine Price is obtained through a variety of data sources. The Deutetrabenazine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Deutetrabenazine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Deutetrabenazine, including repackagers and relabelers. The FDA regulates Deutetrabenazine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Deutetrabenazine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Deutetrabenazine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Deutetrabenazine supplier is an individual or a company that provides Deutetrabenazine active pharmaceutical ingredient (API) or Deutetrabenazine finished formulations upon request. The Deutetrabenazine suppliers may include Deutetrabenazine API manufacturers, exporters, distributors and traders.
click here to find a list of Deutetrabenazine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Deutetrabenazine DMF (Drug Master File) is a document detailing the whole manufacturing process of Deutetrabenazine active pharmaceutical ingredient (API) in detail. Different forms of Deutetrabenazine DMFs exist exist since differing nations have different regulations, such as Deutetrabenazine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Deutetrabenazine DMF submitted to regulatory agencies in the US is known as a USDMF. Deutetrabenazine USDMF includes data on Deutetrabenazine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Deutetrabenazine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Deutetrabenazine suppliers with USDMF on PharmaCompass.
A Deutetrabenazine written confirmation (Deutetrabenazine WC) is an official document issued by a regulatory agency to a Deutetrabenazine manufacturer, verifying that the manufacturing facility of a Deutetrabenazine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Deutetrabenazine APIs or Deutetrabenazine finished pharmaceutical products to another nation, regulatory agencies frequently require a Deutetrabenazine WC (written confirmation) as part of the regulatory process.
click here to find a list of Deutetrabenazine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Deutetrabenazine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Deutetrabenazine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Deutetrabenazine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Deutetrabenazine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Deutetrabenazine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Deutetrabenazine suppliers with NDC on PharmaCompass.
Deutetrabenazine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Deutetrabenazine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Deutetrabenazine GMP manufacturer or Deutetrabenazine GMP API supplier for your needs.
A Deutetrabenazine CoA (Certificate of Analysis) is a formal document that attests to Deutetrabenazine's compliance with Deutetrabenazine specifications and serves as a tool for batch-level quality control.
Deutetrabenazine CoA mostly includes findings from lab analyses of a specific batch. For each Deutetrabenazine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Deutetrabenazine may be tested according to a variety of international standards, such as European Pharmacopoeia (Deutetrabenazine EP), Deutetrabenazine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Deutetrabenazine USP).

