



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
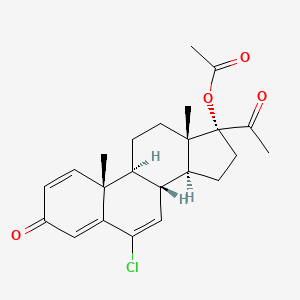

1. Delmadinone
2. Rs1301
3. Tardak
1. 13698-49-2
2. Delmadinone Acetate [usan]
3. [(8r,9s,10r,13s,14s,17r)-17-acetyl-6-chloro-10,13-dimethyl-3-oxo-9,11,12,14,15,16-hexahydro-8h-cyclopenta[a]phenanthren-17-yl] Acetate
4. Rs-1301
5. 6-chloro-17-hydroxypregna-1,4,6-triene-3,20-dione Acetate
6. 92833m0ld9
7. Tardastrex
8. Pregna-1,4,6-triene-3,20-dione, 17-(acetyloxy)-6-chloro-
9. Delmadinone Acetate (usan)
10. Estrex
11. Tardak
12. Cyproterone Impurity I
13. Rs 1301
14. Delmadinoneacetate
15. Unii-92833m0ld9
16. 6-chloro-3,20-dioxopregna-1,4,6-trien-17-yl Acetate (delmadinone Acetate; 1,2-didehydrochlormadinone Acetate)
17. Einecs 237-219-8
18. Schembl146080
19. Chembl2104598
20. Delmadinone Acetate [mi]
21. Niosh/tu4521000
22. Dtxsid60160018
23. Chebi:135645
24. Delmadinone Acetate [mart.]
25. Zinc4215535
26. Tu45210000
27. D03675
28. Q5254243
29. 6-chloro-delta(sup 1,6)-bis-dehydro-17-alpha-acetoxyprogesterone
30. 6-chloro-17-alpha-hydroxypregna-1,4,6-triene-3,20-dione Acetate (ester)
31. Pregna-1,4,6-triene-3,20-dione, 6-chloro-17-alpha-hydroxy-, Acetate (ester)
32. Acetic Acid (8r,9s,10r,13s,14s,17r)-17-acetyl-6-chloro-10,13-dimethyl-3-oxo-8,9,10,11,12,13,14,15,16,17-decahydro-3h-cyclopenta[a]phenanthren-17-yl Ester
| Molecular Weight | 402.9 g/mol |
|---|---|
| Molecular Formula | C23H27ClO4 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 402.1597870 g/mol |
| Monoisotopic Mass | 402.1597870 g/mol |
| Topological Polar Surface Area | 60.4 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 868 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Androgen Antagonists
Compounds which inhibit or antagonize the biosynthesis or actions of androgens. (See all compounds classified as Androgen Antagonists.)
Progestins
Compounds that interact with PROGESTERONE RECEPTORS in target tissues to bring about the effects similar to those of PROGESTERONE. Primary actions of progestins, including natural and synthetic steroids, are on the UTERUS and the MAMMARY GLAND in preparation for and in maintenance of PREGNANCY. (See all compounds classified as Progestins.)


