



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
0
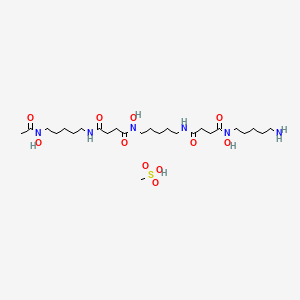

1. Deferoxamine
2. Deferoxamine B
3. Deferoxamine Mesilate
4. Deferoxamine Methanesulfonate
5. Deferoximine
6. Deferrioxamine B
7. Desferal
8. Desferioximine
9. Desferrioxamine
10. Desferrioxamine B
11. Desferrioxamine B Mesylate
12. Desferroxamine
13. Mesilate, Deferoxamine
14. Mesylate, Deferoxamine
15. Mesylate, Desferrioxamine B
16. Methanesulfonate, Deferoxamine
1. 138-14-7
2. Desferal
3. Desferrioxamine B Mesylate
4. Deferoxamine Mesilate
5. Desferrioxamine Mesylate
6. Deferoxamine Methanesulfonate
7. Deferoxamine B Mesylate
8. Deferoxamine (mesylate)
9. Desferal Mesylate
10. Desferrioxamine Mesilate
11. V9tko7eo6k
12. Nsc-756718
13. Mls000028713
14. Desferrioxamine B Mesylate;dfom
15. Ba-33112
16. N-(5-(3-((5-aminopentyl)hydroxycarbamoyl)propionamido)pentyl)-3-((5-(n-hydroxyacetamido)pentyl)carbamoyl)propionohydroxamic Acid Monomethanesulfonate (salt)
17. Ncgc00017021-01
18. Cas-138-14-7
19. Deferoxaminemesylate
20. Desferal (tn)
21. Deferoxamine-d8 Mesylate
22. Deferoxamine Mesylate Salt
23. Dsstox_cid_17649
24. Dsstox_rid_79351
25. Dsstox_gsid_37649
26. N-[5-[[4-[5-[acetyl(hydroxy)amino]pentylamino]-4-oxobutanoyl]-hydroxyamino]pentyl]-n'-(5-aminopentyl)-n'-hydroxybutanediamide;methanesulfonic Acid
27. Desferal Methanesulfonate
28. Chebi:31460
29. Deferoxamine Mesylate [usan]
30. Nsc644468
31. Desferioxamine Mesylate
32. Sr-01000695424
33. Smr000058548
34. Einecs 205-314-3
35. Unii-v9tko7eo6k
36. Ba 33122
37. 138d147
38. Ccris 8311
39. Deferoxamine Mesylate [usan:usp]
40. Deferoxamini Mesilas
41. Prestwick_988
42. Mfcd00058605
43. Chembl1234
44. Deferoxamine Mesylate (usp)
45. Schembl119982
46. Spectrum1500224
47. Deferoxamine Mesilate (jp17)
48. Dtxsid6037649
49. Hms500e04
50. Hms1570a12
51. Hms1920c16
52. Hms2091k08
53. Hms2097a12
54. Hms2234h23
55. Hms3714a12
56. Pharmakon1600-01500224
57. Deferoxamine Mesilate [jan]
58. Bcp31290
59. Ex-a4085
60. Hy-b0988
61. Tox21_110741
62. Ccg-39770
63. Deferoxamine Mesylate [vandf]
64. Nsc756718
65. S5742
66. Akos026750165
67. Deferoxamine Mesilate [who-dd]
68. Deferoxamine Mesilate [who-ip]
69. Deferoxamine Mesylate [usp-rs]
70. Tox21_110741_1
71. Cs-4479
72. Nsc 756718
73. Nsc-644468
74. Desferrioxamine Mesilate [mart.]
75. Desferrioxamine Mesylate [who-ip]
76. Ncgc00017021-02
77. Ncgc00017021-03
78. Ncgc00094640-01
79. Ncgc00094640-02
80. Ncgc00178802-06
81. Ac-36517
82. Butanediamide, N'-(5-((4-((5-(acetylhydroxyamino)pentyl)amino)-1,4-dioxobutyl)hydroxyamino)pentyl)-n-(5-aminopentyl)-n-hydroxy-, Monomethanesulfonate
83. Deferoxamine Mesylate - Cas 138-14-7
84. Deferoxamine Methanesulfonate [mi]
85. Propionohydroxamic Acid, N-(5-(3-((5-aminopentyl)hydroxycarbamoyl)propionamido)pentyl)-3-((5-(n-hydroxyacetamido)pentyl)carbamoyl)-, Monomethanesulfonate (salt)
86. Deferoxamine Mesylate [orange Book]
87. Db-042415
88. Deferoxamine Mesilate [ep Monograph]
89. Deferoxamini Mesilas [who-ip Latin]
90. Deferoxamine Mesylate [usp Monograph]
91. Ft-0603121
92. D01186
93. A807339
94. Sr-01000695424-2
95. Sr-01000695424-3
96. Sr-01000695424-4
97. Deferoxamine Mesylate Salt, Powder, >=92.5% (tlc)
98. Q27114315
99. Desferrioxamine B Mesylate; Dfom;desferrioxamine Mesylate
100. Deferoxamine Mesylate Salt, European Pharmacopoeia (ep) Reference Standard
101. Deferoxamine Mesylate, United States Pharmacopeia (usp) Reference Standard
102. Deferoxamine For System Suitability, European Pharmacopoeia (ep) Reference Standard
103. Butanediamide, N'(-(((((acetylhydroxyamino)pentyl)mino)1,4-dioxobutyl)ydroxyamino)entyl)n-(5-aminopentyl)-n-hydroxy-, Monomethanesulfonate
104. Butanediamide, N'(-(((((acetylhydroxyamino)pentyl)mino)1,4-dioxobutyl)ydroxyamino)entyl)n-(5-aminopentyl)-n-hydroxy-, Monomethanesulphonate
105. N'-(5-azanylpentyl)-n-[5-[[4-[5-[ethanoyl(oxidanyl)amino]pentylamino]-4-oxidanylidene-butanoyl]-oxidanyl-amino]pentyl]-n'-oxidanyl-butanediamide; Methanesulfonic Acid
106. N'-{5-[acetyl(hydroxy)amino]pentyl}-n-[5-({4-[(5-aminopentyl)(hydroxy)amino]-4-oxobutanoyl}amino)pentyl]-n-hydroxysuccinamide Methanesulfonate (salt)
107. N-(5-(3-((5-aminopentyl)hydroxycarbamoyl)propionamido)pentyl)-3-((5-(n-hydroxyacetamido)pentyl)carbamoyl)propionohydroxamic Acid Monomethanesulphonate (salt)
108. N-[5-[[4-[5-[acetyl(hydroxy)amino]pentylamino]-1,4-dioxobutyl]-hydroxyamino]pentyl]-n'-(5-aminopentyl)-n'-hydroxybutanediamide; Methanesulfonic Acid
109. N1-(5-(4-((5-aminopentyl)amino)-4-oxobutanamido)pentyl)-n1-hydroxy-n4-(5-(n-hydroxyacetamido)pentyl)succinamide Methanesulfonate
110. N1-(5-aminopentyl)-n1-hydroxy-n4-(5-(n-hydroxy-4-(5-(n-hydroxyacetamido)pentylamino)-4-oxobutanamido)pentyl)succinamide Methanesulfonate
111. N4-[5-[[4-[[5-(acetylhydroxyamino)pentyl]amino-1,4-dioxobutyl]hydroxyamino]pentyl]-n1-(5-aminopentyl)-n1-hydroxybutanediamide Methanesulfonate
| Molecular Weight | 656.8 g/mol |
|---|---|
| Molecular Formula | C26H52N6O11S |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 23 |
| Exact Mass | 656.34147767 g/mol |
| Monoisotopic Mass | 656.34147767 g/mol |
| Topological Polar Surface Area | 269 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 832 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Deferoxamine mesylate |
| Drug Label | Deferoxamine mesylate for injection, USP, is an iron-chelating agent, available in vials for intramuscular, subcutaneous, and intravenous administration. Deferoxamine mesylate is supplied as vials containing 500 mg and 2 g of deferoxamine mesylate US... |
| Active Ingredient | Deferoxamine mesylate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/vial; 2gm/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Hospira; Eurohlth Intl |
| 2 of 4 | |
|---|---|
| Drug Name | Desferal |
| PubMed Health | Deferoxamine (Injection) |
| Drug Classes | Heavy Metal Chelator |
| Drug Label | Desferal, deferoxamine mesylate USP, is an iron-chelating agent, available in vials for intramuscular, subcutaneous, and intravenous administration. Desferal is supplied as vials containing 500 mg and 2 g of deferoxamine mesylate USP in sterile, lyop... |
| Active Ingredient | Deferoxamine mesylate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/vial; 2gm/vial |
| Market Status | Prescription |
| Company | Novartis |
| 3 of 4 | |
|---|---|
| Drug Name | Deferoxamine mesylate |
| Drug Label | Deferoxamine mesylate for injection, USP, is an iron-chelating agent, available in vials for intramuscular, subcutaneous, and intravenous administration. Deferoxamine mesylate is supplied as vials containing 500 mg and 2 g of deferoxamine mesylate US... |
| Active Ingredient | Deferoxamine mesylate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/vial; 2gm/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Hospira; Eurohlth Intl |
| 4 of 4 | |
|---|---|
| Drug Name | Desferal |
| PubMed Health | Deferoxamine (Injection) |
| Drug Classes | Heavy Metal Chelator |
| Drug Label | Desferal, deferoxamine mesylate USP, is an iron-chelating agent, available in vials for intramuscular, subcutaneous, and intravenous administration. Desferal is supplied as vials containing 500 mg and 2 g of deferoxamine mesylate USP in sterile, lyop... |
| Active Ingredient | Deferoxamine mesylate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/vial; 2gm/vial |
| Market Status | Prescription |
| Company | Novartis |
Siderophores
Low-molecular-weight compounds produced by microorganisms that aid in the transport and sequestration of ferric iron. (The Encyclopedia of Molecular Biology, 1994) (See all compounds classified as Siderophores.)


