



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
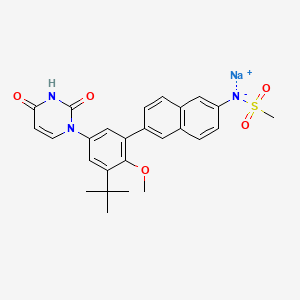

1. 1132940-11-4
2. Dasabuvir Sodium Anhydrous
3. N-(6-(3-(tert-butyl)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2h)-yl)-2-methoxyphenyl)naphthalen-2-yl)methanesulfonamide Sodium Salt
4. Sodium (6-(3-tert-butyl-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2h)-yl)-2-methoxyphenyl)naphthalen-2-yl)(methylsulfonyl)amide
5. Chebi:85179
6. Q27158387
7. Sodium {6-[3-tert-butyl-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2h)-yl)-2-methoxyphenyl]naphthalen-2-yl}(methanesulfonyl)azanide
8. Sodium;[6-[3-tert-butyl-5-(2,4-dioxopyrimidin-1-yl)-2-methoxyphenyl]naphthalen-2-yl]-methylsulfonylazanide
| Molecular Weight | 515.6 g/mol |
|---|---|
| Molecular Formula | C26H26N3NaO5S |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 515.14908639 g/mol |
| Monoisotopic Mass | 515.14908639 g/mol |
| Topological Polar Surface Area | 102 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 944 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Exviera is indicated in combination with other medicinal products for the treatment of chronic hepatitis C (CHC) in adults.
For hepatitis C virus (HCV) genotype specific activity.
J05AP09




