



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
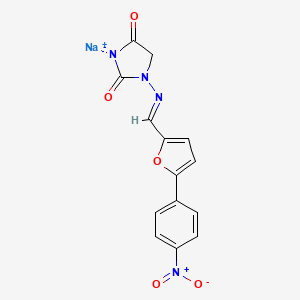

1. Dantrium
2. Dantrolene
3. Dantrolene Sodium
4. Sodium, Dantrolene
1. Dantrolene Sodium
2. 14663-23-1
3. Tox21_500424
4. Ccg-221728
5. Ncgc00261109-01
6. Ac-25752
7. D 9175
| Molecular Weight | 336.23 g/mol |
|---|---|
| Molecular Formula | C14H9N4NaO5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Exact Mass | 336.04706368 g/mol |
| Monoisotopic Mass | 336.04706368 g/mol |
| Topological Polar Surface Area | 110 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 530 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Dantrolene sodium |
| Drug Label | The chemical formula of dantrolene sodium is hydrated 1-[[[5-(4-nitrophenyl)-2-furanyl]methylene]amino]-2, 4-imidazolidinedione sodium salt. It is an orange powder, slightly soluble in water, but due to its slightly acidic nature the solubility incre... |
| Active Ingredient | Dantrolene sodium |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 100mg; 25mg; 50mg |
| Market Status | Prescription |
| Company | Mikah Pharma; Impax Labs |
| 2 of 2 | |
|---|---|
| Drug Name | Dantrolene sodium |
| Drug Label | The chemical formula of dantrolene sodium is hydrated 1-[[[5-(4-nitrophenyl)-2-furanyl]methylene]amino]-2, 4-imidazolidinedione sodium salt. It is an orange powder, slightly soluble in water, but due to its slightly acidic nature the solubility incre... |
| Active Ingredient | Dantrolene sodium |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 100mg; 25mg; 50mg |
| Market Status | Prescription |
| Company | Mikah Pharma; Impax Labs |
Muscle Relaxants, Central
A heterogeneous group of drugs used to produce muscle relaxation, excepting the neuromuscular blocking agents. They have their primary clinical and therapeutic uses in the treatment of muscle spasm and immobility associated with strains, sprains, and injuries of the back and, to a lesser degree, injuries to the neck. They have been used also for the treatment of a variety of clinical conditions that have in common only the presence of skeletal muscle hyperactivity, for example, the muscle spasms that can occur in MULTIPLE SCLEROSIS. (From Smith and Reynard, Textbook of Pharmacology, 1991, p358) (See all compounds classified as Muscle Relaxants, Central.)








