

X


API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
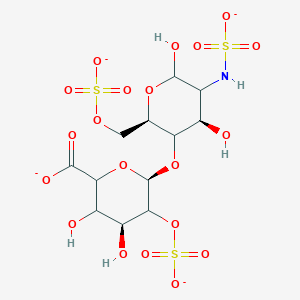

| Molecular Weight | 591.5 g/mol |
|---|---|
| Molecular Formula | C12H17NO20S3-4 |
| XLogP3 | -5.6 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 21 |
| Rotatable Bond Count | 6 |
| Exact Mass | 590.95060545 g/mol |
| Monoisotopic Mass | 590.95060545 g/mol |
| Topological Polar Surface Area | 376 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | -4 |
| Complexity | 1040 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 6 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Heparin sodium in plastic container |
| Active Ingredient | Heparin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10,000 units/ml; 5,000 units/ml; 20,000 units/ml; 1,000 units/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 2 of 4 | |
|---|---|
| Drug Name | Heparin sodium preservative free |
| Active Ingredient | Heparin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10,000 units/ml; 1,000 units/ml |
| Market Status | Prescription |
| Company | Hospira; Shenzhen Techdow; Pfizer; Fresenius Kabi Usa; Sagent Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Heparin sodium in plastic container |
| Active Ingredient | Heparin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10,000 units/ml; 5,000 units/ml; 20,000 units/ml; 1,000 units/ml |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa |
| 4 of 4 | |
|---|---|
| Drug Name | Heparin sodium preservative free |
| Active Ingredient | Heparin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10,000 units/ml; 1,000 units/ml |
| Market Status | Prescription |
| Company | Hospira; Shenzhen Techdow; Pfizer; Fresenius Kabi Usa; Sagent Pharms |




