



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
0
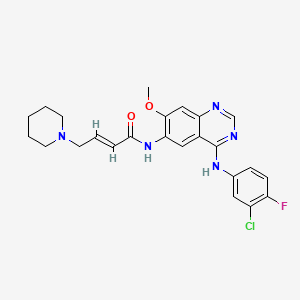

1. N-(4-(3-chloro-4-fluoroanilino)-7-methoxy-6-quinazolinyl)-4-(1-piperidinyl)-2-butenamide
2. Pf 00299804
3. Pf-00299804
4. Pf00299804
5. Vizimpro
1. 1110813-31-4
2. Pf-00299804
3. Pf299804
4. Dacomitinib (pf299804, Pf299)
5. Dacomitinib Anhydrous
6. (2e)-n-[4-[(3-chloro-4-fluorophenyl)amino]-7-methoxy-6-quinazolinyl]-4-(1-piperidinyl)-2-butenamide
7. (e)-n-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-4-(piperidin-1-yl)but-2-enamide
8. Pf-299804
9. Dacomitinib (pf299804)
10. 2xjx250c20
11. Pf 00299804-03
12. Dacomitinib (inn)
13. Dacomitinib [inn]
14. (e)-n-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-yl]-4-piperidin-1-ylbut-2-enamide
15. (2e)-n-{4-[(3-chloro-4-fluorophenyl)amino]-7-methoxyquinazolin-6-yl}-4-(piperidin-1-yl)but-2-enamide
16. Dacomitinib [usan:inn]
17. Dacomitinibum
18. Unii-2xjx250c20
19. Pf299
20. (e)-n-[4-[(3-chloro-4-fluorophenyl)amino]-7-methoxyquinazolin-6-yl]-4-(piperidin-1-yl)but-2-enamide
21. Pf-299
22. Dacomitinib [who-dd]
23. Mls006011275
24. Gtpl7422
25. Chembl2110732
26. Chebi:91466
27. Dtxsid50149493
28. Ex-a030
29. Chebi:132268
30. Bdbm112499
31. Dacomitinib (pf-00299804)
32. Amy21292
33. Bcp02530
34. Mfcd19443734
35. Nsc765888
36. Nsc800084
37. S2727
38. Zinc72266312
39. Akos025401818
40. Ccg-264987
41. Cs-0500
42. Db11963
43. Nsc-765888
44. Nsc-800084
45. Us8623883, No. 2
46. Ncgc00263185-09
47. Ncgc00263185-10
48. Ac-25915
49. As-57686
50. Hy-13272
51. Smr004703025
52. D5450
53. Sw219155-1
54. D09883
55. Dacomitinib (pf299804, Pf-00299804)
56. Pf-299804 (dacomitinib Pf-00299804)
57. J-500784
58. Q17130597
59. (2e)-n-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxyquinazolin-6-yl)-4-piperidin-1-ylbut-2-enamide
60. (2e)-n-[4-(3-chloro-4-fluoroanilino)-7-methoxyquinazolin-6-yl]-4-(piperidin-1-yl)but-2-enamide
61. (e)-n-(4-(3-chloro-4-fluorophenylamino)-7-methoxyquinazolin-6-yl)-4-(piperidin-1-yl)but-2-enamide
62. (e)-n-[4-[(3-chloro-4-fluorophenyl)amino]-7-methoxyquinazolin-6-yl]-4-piperidin-1-ylbut-2-enamide
63. 2-butenamide, N-(4-((3-chloro-4-fluorophenyl)amino)-7-methoxy-6-quinazolinyl)-4-(1-piperidinyl)-, (2e)-
| Molecular Weight | 469.9 g/mol |
|---|---|
| Molecular Formula | C24H25ClFN5O2 |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 7 |
| Exact Mass | 469.1680809 g/mol |
| Monoisotopic Mass | 469.1680809 g/mol |
| Topological Polar Surface Area | 79.4 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 665 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Dacomitinib is indicated as the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutations as verified by an FDA-approved test. Lung cancer is the leading cause of cancer death and NSCLC accounts for 85% of lung cancer cases. From the cases of NSCLC, approximately 75% of the patients present a late diagnosis with metastatic and advanced disease which produces a survival rate of 5%. The presence of a mutation in EGFR accounts for more than the 60% of the NSCLC cases and the overexpression of EGFR is associated with frequent lymph node metastasis and poor chemosensitivity.
FDA Label
Vizimpro, as monotherapy, is indicated for the first-line treatment of adult patients with locally advanced or metastatic non small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) activating mutations.
Preclinical data suggested that dacomitinib increases the inhibition of the epidermal growth factor receptor kinase domain as well as the activity in cell lines harboring resistance mutations such as T790M. This activity further produced a significant reduction of EGFR phosphorylation and cell viability. In these studies, non-small cell lymphoma cancer cell lines with L858R/T790M mutations where used and an IC50 of about 280 nmol/L was observed. In clinical trials with patients with advanced non-small cell lung carcinoma who progressed after chemotherapy, there was an objective response rate of 5% with a progression-free survival of 2.8 months and an overall survival of 9.5 months. As well, phase I/II studies showed positive dacomitinib activity despite prior failure with tyrosine kinase inhibitors. Phase III clinical trials (ARCHER 1050), done in patients suffering from advanced or metastatic non-small cell lung carcinoma with EGFR-activating mutations, reported a significant improvement in progression-free survival when compared with gefitinib.
L01EB07
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EB - Epidermal growth factor receptor (egfr) tyrosine kinase inhibitors
L01EB07 - Dacomitinib
Absorption
Dacomitinib has shown a linear kinetics after single and multiple dose range studies. The absorption and distribution do not seem to be affected by food or the consumption of antacids. The peak plasma concentration after a dosage of 45 mg for 4 days is of 104 ng/ml. The reported AUC0-24h and tmax are of 2213 ng.h/mL and 6 hours, respectively. As well, following oral administration, the absolute oral bioavailability is 80%.
Route of Elimination
From the administered dose, 79% is recovered in feces, from which 20% represents the unmodified form of dacomitinib, and 3% is recovered in urine, from which <1% is represented by the unchanged form.
Volume of Distribution
The volume of distribution of dacomitinib was reported to be of 2415 L.
Clearance
The geometric apparent clearance of dacomitinib is 27.06 L/h.
Dacomitinib presents an oxidative and conjugative metabolism marked mainly by the activity of glutathione and cytochrome P450 enzymes. After metabolism, its major circulating metabolite is an O-desmethyl dacomitinib form named PF-05199265. This metabolite has been shown to be formed by an oxidative step by CYP2D6 and to a smaller extent by CYP2C9. The following steps of the metabolism are mainly mediated by CYP3A4 for the formation of smaller metabolites. From these metabolic studies, it was shown that dacomitinib inhibited strongly the activities of CYP2D6.
Dacomitinib is reported to have a very large half-life of 70 hours.
Dacomitinib is an irreversible small molecule inhibitor of the activity of the human epidermal growth factor receptor (EGFR) family (EGFR/HER1, HER2, and HER4) tyrosine kinases. It achieves irreversible inhibition via covalent bonding to the cysteine residues in the catalytic domains of the HER receptors. The affinity of dacomitinib has been shown to have an IC50 of 6 nmol/L. The ErbB or epidermal growth factor (EGF) family plays a role in tumor growth, metastasis, and treatment resistance by activating downstream signal transduction pathways such as such as Ras-Raf-MAPK, PLCgamma-PKC-NFkB and PI3K/AKT through the tyrosine kinase-driven phosphorylation at the carboxy-terminus. Around 40% of cases show amplification of EGFR gene and 50% of the cases present the _EGFRvIII_ mutation which represents a deletion that produces a continuous activation of the tyrosine kinase domain of the receptor.


