
Synopsis
Synopsis
0
EU WC
0
KDMF
0
VMF
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Amivalex
2. Duphalac
3. Normase
1. D-lactulose
2. Bifiteral
3. Cephulac
4. Chronulac
5. Constilac
6. Cholac
7. Lactulosa
8. Lactulosum
9. Duphalac
10. 4618-18-2
11. 4-o-beta-d-galactopyranosyl-d-fructose
12. 9xh2p2n8ep
13. 4-o-beta-d-galactopyranosyl-d-fructofuranose
14. Chebi:6359
15. 58166-24-8
16. Isolactose
17. Laevolac
18. 4-o-beta-d-galactopyranosyl-beta-d-fructofuranose
19. Nsc-757082
20. (2s,3r,4s,5r,6r)-2-[(2r,3s,4s,5r)-4,5-dihydroxy-2,5-bis(hydroxymethyl)oxolan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol
21. Lactulosum [latin]
22. Lactulosa [spanish]
23. Kristalose
24. Lattulosio [italian]
25. Lactulosum [inn-latin]
26. Dsstox_cid_25833
27. Dsstox_rid_81161
28. Dsstox_gsid_45833
29. Lactulosa [inn-spanish]
30. 58166-25-9
31. Lattulosio
32. Actilax
33. Alpha-lactulose
34. Cephulac (tn)
35. (2s,3r,4s,5r,6r)-2-(((2r,3s,4s,5r)-4,5-dihydroxy-2,5-bis(hydroxymethyl)tetrahydrofuran-3-yl)oxy)-6-(hydroxymethyl)tetrahydro-2h-pyran-3,4,5-triol
36. Cas-4618-18-2
37. Sr-05000002084
38. Einecs 225-027-7
39. Unii-9u7d5qh5ae
40. Unii-9xh2p2n8ep
41. Brn 0093773
42. Brn 6278818
43. 4-beta-d-galactosido-(1,4)-d-fructose
44. Delta-lactulose
45. D-fructose, 4-o-beta-d-galactopyranosyl-
46. Cephulac Syrup
47. Lactulose Syrup
48. 4-o-b-d-galactopyranosyl-d-fructose
49. Lactulose Jp17
50. Chronulac (tn)
51. Ncgc00094707-01
52. W9t
53. Beta-d-fructofuranose, 4-o-beta-d-galactopyranosyl-
54. Lactulose [usan:usp:inn:ban:jan]
55. 4-o-beta-d-galattopiranosil-d-fruttofuranosio [italian]
56. Spectrum_000857
57. Spectrum2_001159
58. Spectrum3_000478
59. Spectrum4_000962
60. Spectrum5_000908
61. .alpha.-lactulose
62. 4-o-beta-d-galattopiranosil-d-fruttofuranosio
63. Schembl18912
64. Bspbio_002216
65. Kbiogr_001303
66. Kbioss_001337
67. 5-17-07-00214 (beilstein Handbook Reference)
68. Fructofuranose, 4-o-beta-d-galactopyranosyl-, D-
69. Divk1c_000064
70. Spectrum1500363
71. Spbio_001117
72. Lactulose (jp17/usp/inn)
73. Alpha-d-fructofuranose, 4-o-beta-d-galactopyranosyl-
74. Chembl296306
75. Dtxsid5045833
76. Hms500d06
77. Kbio1_000064
78. Kbio2_001337
79. Kbio2_003905
80. Kbio2_006473
81. Kbio3_001436
82. Ninds_000064
83. Hms1920j03
84. Hms2094g15
85. Pharmakon1600-01500363
86. Zinc3977952
87. Tox21_111318
88. Bdbm50377984
89. Ccg-39552
90. Mfcd00151469
91. Nsc757082
92. Akos024283994
93. Tox21_111318_1
94. Db00581
95. Nsc 757082
96. Idi1_000064
97. Ncgc00142624-01
98. Ncgc00142624-02
99. Ncgc00142624-03
100. Ncgc00142624-05
101. 2-[(3s,4s,2r,5r)-2,5-bis(hydroxymethyl)-4,5-dihydroxyoxolan-3-yloxy](2s,4s,3r, 5r,6r)-6-(hydroxymethyl)-2h-3,4,5,6-tetrahydropyran-3,4,5-triol
102. 4-o-b-d-galactopyranosyl-d-fructofuranose
103. 4-o-beta-delta-galactopyranosyl-delta-fructose
104. C07064
105. D00352
106. Ab00052029_02
107. Q422689
108. 4-o-beta-delta-galactopyranosyl-delta-fructofuranose
109. Sr-05000002084-1
110. Sr-05000002084-4
111. .alpha.-d-fructofuranose, 4-o-.beta.-d-galactopyranosyl-
112. Wurcs=2.0/2,2,1/[ha122h-2b_2-5][a2112h-1b_1-5]/1-2/a4-b1
113. (2s,3r,4s,5r,6r)-2-{[(2r,3s,4s,5r)-4,5-dihydroxy-2,5-bis(hydroxymethyl)oxolan-3-yl]oxy}-6-(hydroxymethyl)oxane-3,4,5-triol
| Molecular Weight | 342.30 g/mol |
|---|---|
| Molecular Formula | C12H22O11 |
| XLogP3 | -4.3 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 5 |
| Exact Mass | 342.11621151 g/mol |
| Monoisotopic Mass | 342.11621151 g/mol |
| Topological Polar Surface Area | 190 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 395 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 12 | |
|---|---|
| Drug Name | Cholac |
| PubMed Health | Lactulose (By mouth) |
| Drug Classes | Gastrointestinal Agent, Laxative, Hyperosmotic |
| Drug Label | Generlac Solution (Lactulose Solution, USP) is a synthetic disaccharide in solution form for oral or rectal administration. Each 15 mL of Generlac Solution contains: 10 g lactulose (and not more than 1.6 g galactose, not more than 1.2 g lactose, not... |
| Active Ingredient | Lactulose |
| Dosage Form | Solution |
| Route | Oral, rectal |
| Strength | 10gm/15ml |
| Market Status | Prescription |
| Company | Alra |
| 2 of 12 | |
|---|---|
| Drug Name | Constilac |
| Active Ingredient | Lactulose |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 10gm/15ml |
| Market Status | Prescription |
| Company | Alra |
| 3 of 12 | |
|---|---|
| Drug Name | Constulose |
| PubMed Health | Lactulose (By mouth) |
| Drug Classes | Gastrointestinal Agent, Laxative, Hyperosmotic |
| Drug Label | Lactulose is a synthetic disaccharide in solution form for oral administration. Each 15 mL of lactulose solution contains: 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 0.1 g or less of fructose).Lactulose is a colonic a... |
| Active Ingredient | Lactulose |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 10gm/15ml |
| Market Status | Prescription |
| Company | Actavis Mid Atlantic |
| 4 of 12 | |
|---|---|
| Drug Name | Enulose |
| PubMed Health | Lactulose (By mouth) |
| Drug Classes | Gastrointestinal Agent, Laxative, Hyperosmotic |
| Drug Label | Lactulose is a synthetic disaccharide in solution form for oral or rectal administration. Each15 mL of lactulose solution contains 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 0.1 g or less of fructose). Lactulose solut... |
| Active Ingredient | Lactulose |
| Dosage Form | Solution |
| Route | Oral, rectal |
| Strength | 10gm/15ml |
| Market Status | Prescription |
| Company | Actavis Mid Atlantic |
| 5 of 12 | |
|---|---|
| Drug Name | Generlac |
| PubMed Health | Lactulose (By mouth) |
| Drug Classes | Gastrointestinal Agent, Laxative, Hyperosmotic |
| Drug Label | Generlac Solution (Lactulose Solution, USP) is a synthetic disaccharide in solution form for oral or rectal administration. Each 15 mL of Generlac Solution contains: 10 g lactulose (and not more than 1.6 g galactose, not more than 1.2 g lactose, not... |
| Active Ingredient | Lactulose |
| Dosage Form | Solution |
| Route | Oral, rectal |
| Strength | 10gm/15ml |
| Market Status | Prescription |
| Company | Morton Grove Pharms |
| 6 of 12 | |
|---|---|
| Drug Name | Lactulose |
| Active Ingredient | Lactulose |
| Dosage Form | For solution; Solution |
| Route | Oral; Oral, rectal |
| Strength | 10gm/packet; 10gm/15ml; 20gm/packet |
| Market Status | Prescription |
| Company | Vintage Pharms; Ani Pharms; Fresenius Kabi; Pharm Assoc; Morton Grove; Roxane; Cumberland Pharms; Vistapharm; Hi Tech Pharma |
| 7 of 12 | |
|---|---|
| Drug Name | Cholac |
| PubMed Health | Lactulose (By mouth) |
| Drug Classes | Gastrointestinal Agent, Laxative, Hyperosmotic |
| Drug Label | Generlac Solution (Lactulose Solution, USP) is a synthetic disaccharide in solution form for oral or rectal administration. Each 15 mL of Generlac Solution contains: 10 g lactulose (and not more than 1.6 g galactose, not more than 1.2 g lactose, not... |
| Active Ingredient | Lactulose |
| Dosage Form | Solution |
| Route | Oral, rectal |
| Strength | 10gm/15ml |
| Market Status | Prescription |
| Company | Alra |
| 8 of 12 | |
|---|---|
| Drug Name | Constilac |
| Active Ingredient | Lactulose |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 10gm/15ml |
| Market Status | Prescription |
| Company | Alra |
| 9 of 12 | |
|---|---|
| Drug Name | Constulose |
| PubMed Health | Lactulose (By mouth) |
| Drug Classes | Gastrointestinal Agent, Laxative, Hyperosmotic |
| Drug Label | Lactulose is a synthetic disaccharide in solution form for oral administration. Each 15 mL of lactulose solution contains: 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 0.1 g or less of fructose).Lactulose is a colonic a... |
| Active Ingredient | Lactulose |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 10gm/15ml |
| Market Status | Prescription |
| Company | Actavis Mid Atlantic |
| 10 of 12 | |
|---|---|
| Drug Name | Enulose |
| PubMed Health | Lactulose (By mouth) |
| Drug Classes | Gastrointestinal Agent, Laxative, Hyperosmotic |
| Drug Label | Lactulose is a synthetic disaccharide in solution form for oral or rectal administration. Each15 mL of lactulose solution contains 10 g lactulose (and less than 1.6 g galactose, less than 1.2 g lactose, and 0.1 g or less of fructose). Lactulose solut... |
| Active Ingredient | Lactulose |
| Dosage Form | Solution |
| Route | Oral, rectal |
| Strength | 10gm/15ml |
| Market Status | Prescription |
| Company | Actavis Mid Atlantic |
| 11 of 12 | |
|---|---|
| Drug Name | Generlac |
| PubMed Health | Lactulose (By mouth) |
| Drug Classes | Gastrointestinal Agent, Laxative, Hyperosmotic |
| Drug Label | Generlac Solution (Lactulose Solution, USP) is a synthetic disaccharide in solution form for oral or rectal administration. Each 15 mL of Generlac Solution contains: 10 g lactulose (and not more than 1.6 g galactose, not more than 1.2 g lactose, not... |
| Active Ingredient | Lactulose |
| Dosage Form | Solution |
| Route | Oral, rectal |
| Strength | 10gm/15ml |
| Market Status | Prescription |
| Company | Morton Grove Pharms |
| 12 of 12 | |
|---|---|
| Drug Name | Lactulose |
| Active Ingredient | Lactulose |
| Dosage Form | For solution; Solution |
| Route | Oral; Oral, rectal |
| Strength | 10gm/packet; 10gm/15ml; 20gm/packet |
| Market Status | Prescription |
| Company | Vintage Pharms; Ani Pharms; Fresenius Kabi; Pharm Assoc; Morton Grove; Roxane; Cumberland Pharms; Vistapharm; Hi Tech Pharma |
Lactulose is indicated for use as a laxative in the treatment of chronic constipation in adults and geriatric patients. Additionally, lactulose is also employed as an adjunct to protein restriction and supportive therapy for the prevention and treatment of portal-systemic encephalopathy (PSE), including both the hepatic pre-coma and coma variations. In particular, lactulose solution has been effective at managing PSE resulting from surgical portacaval shunts or from chronic hepatic diseases like cirrhosis. Moreover, there have also been studies demonstrating the capacity for lactulose to minimize the formation of gallstones and even some investigations regarding the experimental use of the agent in developing novel anticancer agents owing to its ability to bind galactin carbohydrates involved in various tumor progressions.
FDA Label
Lactulose formulations are most commonly administered via the oral route or the rectal route. Consequently, because the substance experiences minimal absorption by the gut it typically remains localized in the gastrointestinal tract environment and ultimately demonstrates almost all of its pharmacologic effects within the gut. In particular, as lactulose elicits its laxative effects in enhancing stool amounts and softening stool, such biochemical and physiologic activities can cause increased bowel sounds (borborygmi), a feeling of bloatedness, belching, frequent flatus, and diarrhea.
Gastrointestinal Agents
Drugs used for their effects on the gastrointestinal system, as to control gastric acidity, regulate gastrointestinal motility and water flow, and improve digestion. (See all compounds classified as Gastrointestinal Agents.)
A06AD11
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AD - Osmotically acting laxatives
A06AD11 - Lactulose
Absorption
After administration by the oral route, less than 3% of the given dose of lactulose solution is absorbed by the small intestine. The remaining unabsorbed lactulose reaches the large intestine where it is metabolized - but even then, negligible quantities of unchanged lactulose or its metabolites are absorbed across the colon.
Route of Elimination
The renal excretion of any lactulose that manages to be absorbed into the circulation has been determined to be 3% or less and is generally complete within 24 hours. Any unabsorbed lactulose is largely excreted with stool.
Volume of Distribution
Negligible amounts of lactulose - metabolized or non-metabolized - are absorbed into the body.. Most lactulose that is administered subsequently remains predominantly around the gastrointestinal tract area.
Clearance
Negligible amounts of lactulose - metabolized or non-metabolized - are absorbed into the body.. Regardless, data regarding the clearance of lactulose is not readily available or accessible.
Lactulose is essentially only metabolized in the colon by saccharolytic bacteria that are present there. In particular, the substance is broken down into lactic acid and small amounts of acetic and formic acid. Specific examples of bacteria that normally inhabit the large intestine that are capable of lactulose metabolism include Lactobacilli, Bacteroides, Escherichia coli, and Clostridia.
The data regarding the half-life of lactulose is not readily available or accessible.
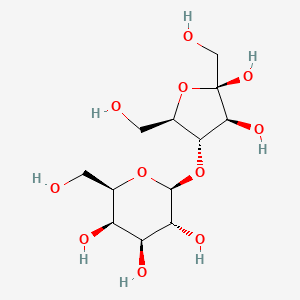
Lactulose is a synthetic disaccharide derivative of lactose that consists of one molecule of galactose and one molecule of fructose. Saccharolytic bacteria present in the large intestine subsequently break the substance down into organic acids like lactic acid and small amounts of formic and acetic acids. Such resultant volatile fatty acid metabolites, in combination with hydrogen and methane that is also generated consequently enhance intraluminal gas formation, peristaltic gut motility, and elicit an osmotic effect that facilitates an increase in the water content of stool as well as associated stool softening. All of these actions ultimately assist in facilitating and increasing the frequency of bowel movements in patients experiencing constipation, although it may take 24 to 48 hours after using the medication for this laxative effect to become evident. At the same time, the formation of such acids via the metabolism of lactulose by colonic bacteria also acidifies the contents of the colon, thereby contributing to the treatment of portal-systemic encephalopathy (PSE). As one of the principal features of PSE involves the accumulation of nitrogenous waste products like ammonia in the systemic circulation, a state in which the colonic contents become more acidic than blood allows ammonia in the circulation to diffuse into the colon.. Furthermore, ammonia that diffuses into the acidic colon is ionized to ammonium ions that are incapable of being absorbed back into the blood. These effects, combined with the laxative action of lactulose facilitates the excretion of excess ammonia. And finally, it is also believed that an acidic colonic environment results in the elimination of urease-producing bacteria that contribute to the formation of ammonia while surviving colonic bacteria use up any trapped ammonia in the colon as a source of nitrogen for protein synthesis.
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1997-060 - Rev 04
Status : Valid
Issue Date : 2010-06-10
Type : Chemical
Substance Number : 924

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Lactulose, Liquid, 634 G/l To 700 G/l
Certificate Number : R1-CEP 2005-102 - Rev 05
Status : Valid
Issue Date : 2020-02-17
Type : Chemical
Substance Number : 924

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Lactulose, Liquid, 620 G/l To 700 G/l
Certificate Number : R1-CEP 2002-162 - Rev 02
Status : Valid
Issue Date : 2013-11-28
Type : Chemical
Substance Number : 924

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2002-236 - Rev 03
Status : Valid
Issue Date : 2017-10-11
Type : Chemical
Substance Number : 1230

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2005-023 - Rev 02
Status : Valid
Issue Date : 2017-09-07
Type : Chemical
Substance Number : 924

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 1998-130 - Rev 03
Status : Valid
Issue Date : 2007-11-30
Type : Chemical
Substance Number : 924

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Lactulose, Liquid, Process II, 670 G/l
Certificate Number : R0-CEP 2019-324 - Rev 00
Status : Expired
Issue Date : 2020-05-19
Type : Chemical and TSE
Substance Number : 924

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Lactulose, Liquid, 63.4% -70.0% W/v
Certificate Number : R1-CEP 1999-145 - Rev 02
Status : Withdrawn by Holder
Issue Date : 2014-05-13
Type : Chemical
Substance Number : 924

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R0-CEP 2001-238 - Rev 01
Status : Expired
Issue Date : 2004-11-15
Type : Chemical
Substance Number : 1230

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Lactulose, Liquid, 670g/l, 695g/l, 720g/l
Certificate Number : R1-CEP 1998-021 - Rev 01
Status : Withdrawn by EDQM Failure to CEP pro...
Issue Date : 2008-03-25
Type : Chemical
Substance Number : 924

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] 
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
78
PharmaCompass offers a list of Lactulose API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Lactulose manufacturer or Lactulose supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Lactulose manufacturer or Lactulose supplier.
PharmaCompass also assists you with knowing the Lactulose API Price utilized in the formulation of products. Lactulose API Price is not always fixed or binding as the Lactulose Price is obtained through a variety of data sources. The Lactulose Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A D-Lactulose manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of D-Lactulose, including repackagers and relabelers. The FDA regulates D-Lactulose manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. D-Lactulose API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of D-Lactulose manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A D-Lactulose supplier is an individual or a company that provides D-Lactulose active pharmaceutical ingredient (API) or D-Lactulose finished formulations upon request. The D-Lactulose suppliers may include D-Lactulose API manufacturers, exporters, distributors and traders.
click here to find a list of D-Lactulose suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A D-Lactulose DMF (Drug Master File) is a document detailing the whole manufacturing process of D-Lactulose active pharmaceutical ingredient (API) in detail. Different forms of D-Lactulose DMFs exist exist since differing nations have different regulations, such as D-Lactulose USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A D-Lactulose DMF submitted to regulatory agencies in the US is known as a USDMF. D-Lactulose USDMF includes data on D-Lactulose's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The D-Lactulose USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of D-Lactulose suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The D-Lactulose Drug Master File in Japan (D-Lactulose JDMF) empowers D-Lactulose API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the D-Lactulose JDMF during the approval evaluation for pharmaceutical products. At the time of D-Lactulose JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of D-Lactulose suppliers with JDMF on PharmaCompass.
A D-Lactulose CEP of the European Pharmacopoeia monograph is often referred to as a D-Lactulose Certificate of Suitability (COS). The purpose of a D-Lactulose CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of D-Lactulose EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of D-Lactulose to their clients by showing that a D-Lactulose CEP has been issued for it. The manufacturer submits a D-Lactulose CEP (COS) as part of the market authorization procedure, and it takes on the role of a D-Lactulose CEP holder for the record. Additionally, the data presented in the D-Lactulose CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the D-Lactulose DMF.
A D-Lactulose CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. D-Lactulose CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of D-Lactulose suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing D-Lactulose as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for D-Lactulose API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture D-Lactulose as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain D-Lactulose and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a D-Lactulose NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of D-Lactulose suppliers with NDC on PharmaCompass.
D-Lactulose Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of D-Lactulose GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right D-Lactulose GMP manufacturer or D-Lactulose GMP API supplier for your needs.
A D-Lactulose CoA (Certificate of Analysis) is a formal document that attests to D-Lactulose's compliance with D-Lactulose specifications and serves as a tool for batch-level quality control.
D-Lactulose CoA mostly includes findings from lab analyses of a specific batch. For each D-Lactulose CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
D-Lactulose may be tested according to a variety of international standards, such as European Pharmacopoeia (D-Lactulose EP), D-Lactulose JP (Japanese Pharmacopeia) and the US Pharmacopoeia (D-Lactulose USP).

