



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
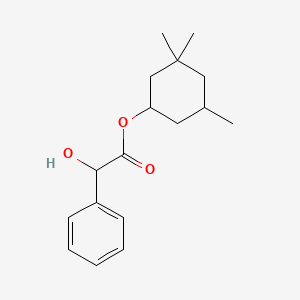

1. Cyclospasmol
1. 456-59-7
2. Cyclomandol
3. Cyclolyt
4. Cyclospasmol
5. Perebral
6. Ciclospasmol
7. Cyclergine
8. Spasmocyclon
9. Spasmocyclone
10. Capilan
11. Clandilon
12. Saiclate
13. Sancyclan
14. Sepyron
15. Spasmione
16. Arto-espasmol
17. 3,3,5-trimethylcyclohexyl Mandelate
18. 3,5,5-trimethylcyclohexyl Mandelate
19. Ciclandelato
20. Cyclobral
21. Novodil
22. Natil
23. 3,5,5-trimethylcyclohexyl Amygdalate
24. Cyclandelatum
25. Cyclandelatum [inn-latin]
26. Ciclandelato [inn-spanish]
27. Mandelic Acid 3,3,5-trimethylcyclohexyl Ester
28. Bs 572
29. 3,3,5-trimethylcyclohexyl 2-hydroxy-2-phenylacetate
30. (3,3,5-trimethylcyclohexyl) 2-hydroxy-2-phenylacetate
31. Spasmocyclon (3m)
32. Cyclandelate (mixture Of Isomers)
33. Mandelic Acid, 3,3,5-trimethylcyclohexyl Ester
34. 3,5,5-trimethylcyclohexanol, Mandelic Acid Ester
35. Nsc-758910
36. Chebi:3988
37. 3,3,5-trimethylcyclohexanol Alpha-phenyl-alpha-hydroxyacetate
38. 3,3,5-trimethylcyclohexyl Hydroxy(phenyl)acetate
39. 4139o1oay2
40. Benzeneacetic Acid, .alpha.-hydroxy-, 3,3,5-trimethylcyclohexyl Ester
41. 3,3,5-trimethylcyclohexyl Mandelate (mixture Of Isomers)
42. Ncgc00159506-02
43. Pericyclon
44. Hacosan
45. Lisospasm
46. Eparan
47. Cyclandelic Acid
48. Rythmol [vasodilator]
49. Hsdb 3046
50. Einecs 207-271-6
51. Mandelic
52. Unii-4139o1oay2
53. Benzeneacetic Acid, Alpha-hydroxy-, 3,3,5-trimethylcyclohexyl Ester
54. Cyclandelate [usp:inn:ban:jan]
55. Mfcd00056623
56. Alpha-hydroxybenzeneacetic Acid 3,3,5-trimethylcyclohexyl Ester
57. Cyclandelate [mi]
58. Cyclospasmol (tn)
59. Dsstox_cid_2862
60. Cyclandelate [inn]
61. Cyclandelate [jan]
62. Cyclandelate [hsdb]
63. Schembl5123
64. Cyclandelate [vandf]
65. Dsstox_rid_76763
66. Dsstox_gsid_22862
67. Cyclandelate [mart.]
68. Cyclandelate [usp-rs]
69. Cyclandelate [who-dd]
70. Cyclandelate (jan/usp/inn)
71. (3,3,5-trimethylcyclohexyl) 2-hydroxy-2-phenyl-acetate
72. Chembl1480987
73. Dtxsid4022862
74. Hms3264k11
75. Hms3652b09
76. Pharmakon1600-01505082
77. Cyclandelate [usp Impurity]
78. Hy-b1170
79. Tox21_111725
80. Nsc758910
81. S4189
82. Zinc00000189
83. Zinc00405331
84. Zinc00968262
85. Akos015902290
86. Ccg-213926
87. Cs-4770
88. Db04838
89. Ks-5317
90. Nsc 758910
91. Ncgc00159506-03
92. Ncgc00159506-05
93. Ncgc00159506-06
94. Cas-456-59-7
95. Sbi-0207036.p001
96. Ft-0735837
97. M0821
98. Sw220003-1
99. D00286
100. D91389
101. Ab01563218_01
102. Ab01563218_02
103. A913217
104. Sr-01000872740
105. 3,3,5-trimethylcyclohexyl Hydroxy(phenyl)acetate #
106. Q1147309
107. Sr-01000872740-1
108. .alpha.-hydroxybenzeneacetic Acid 3,3,5-trimethylcyclohexyl Ester
109. 3,3,5-trimethylcyclohexanol .alpha.-phenyl-.alpha.-hydroxyacetate
| Molecular Weight | 276.4 g/mol |
|---|---|
| Molecular Formula | C17H24O3 |
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 276.17254462 g/mol |
| Monoisotopic Mass | 276.17254462 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 331 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 3 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Vasodilator Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
IT RELAXES VASCULAR SMOOTH MUSCLE & ERRATICALLY INCR BLOOD FLOW. IT IS USED IN TREATMENT OF THROMBOPHLEBITIS, RAYNAUD'S DISEASE, THROMBO-ANGIITIS OBLITERANS, DIABETIC ANGIOSES, INTERMITTENT CLAUDICATION, ARTERIOSCLEROSIS, ERYTHROCYANOSIS, FROSTBITE, & REFRACTORY SKIN ULCERS.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 787
CYCLANDELATE HAS BEEN USED IN ALMOST EVERY KNOWN TYPE OF VASCULAR DISEASE, BUT ITS VALUE HAS NOT BEEN CONVINCINGLY DEMONSTRATED IN ANY.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 736
CYCLANDELATE CAN PRODUCE MILD VASODILATION IN MAN. THIS HAS BEEN NOTED PARTICULARLY AS INCR IN DIGITAL PULSE VOL & SKIN TEMP, BUT INCR IN CEREBRAL & MUSCLE BLOOD FLOW HAVE ALSO BEEN REPORTED.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 736
For more Therapeutic Uses (Complete) data for CYCLANDELATE (7 total), please visit the HSDB record page.
...WHETHER REDUCTION OF PRESSURE IN ARTERIES SUPPLYING OPTIC NERVEHEAD MIGHT RENDER OPTIC NERVE MORE VULNERABLE TO INJURY IN GLAUCOMA REMAINS TO BE EVALUATED.
Grant, W. M. Toxicology of the Eye. 2nd ed. Springfield, Illinois: Charles C. Thomas, 1974., p. 340
Used in the treatment of various blood vessel diseases (e.g., claudication, arteriosclerosis and Raynaud's disease) and nighttime leg cramps.
Cyclandelate is in a class of drugs called vasodilators. Cyclandelate relaxes veins and arteries, which makes them wider and allows blood to pass through them more easily.
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
C - Cardiovascular system
C04 - Peripheral vasodilators
C04A - Peripheral vasodilators
C04AX - Other peripheral vasodilators
C04AX01 - Cyclandelate
Absorption
Well absorbed following oral administration.
MAX UPTAKE @ 1 HR FOR MOST RAT TISSUES WHILE BRAIN, DIAPHRAGM, STOMACH, & VEIN SHOWED MAX LEVELS @ 24 HR. LEVELS NEGLIGIBLE IN 28 DAYS.
ORR A, WHITTIER JR; DISTRIBUTION OF TRITIATED CYCLANDELATE, VASOACTIVE DRUG. PRELIMINARY OBSERVATIONS BY LIQUID SCINTILLATION SPECTROMETRY; INT J NUCL MED BIOL 1(4) 205-213 (1974)
(3-)-H-LABELED CYCLANDELATE WAS DETECTED & QUANTITATED. EFFECTIVE HALF-LIFE VALUES IN VARIOUS RAT TISSUES WERE 2.8-4.1 DAYS.
ORR A, WHITTIER JR; DISTRIBUTION OF TRITIATED CYCLANDELATE, VASOACTIVE DRUG. PRELIMINARY OBSERVATIONS BY LIQUID SCINTILLATION SPECTROMETRY; INT J NUCL MED BIOL 1(4) 205-213 (1974)
Cyclandelate produces peripheral vasodilation by a direct effect on vascular smooth muscle. Pharmacological action may be due to calcium-channel antagonism.
...MOST OF ITS PHARMACOLOGICAL PROPERTIES ARE VERY SIMILAR TO THOSE OF PAPAVERINE & OF MANY SYNTHETIC ANTISPASMODICS PROMOTED PRIMARILY FOR EFFECTS ON SMOOTH MUSCLE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 736
PAPAVERINE IS POTENT INHIBITOR OF CYCLIC NUCLEOTIDE PHOSPHODIESTERASE FOUND IN MANY TISSUES & CAN INCR CONCN OF CYCLIC ADENOSINE 3',5'-MONOPHOSPHATE (CYCLIC AMP). SINCE CYCLIC AMP HAS BEEN IMPLICATED AS POSSIBLE MEDIATOR OF BETA-ADRENERGIC RELAXATION OF SMOOTH MUSCLE, SUCH MECHANISM OF ACTION...IS PLAUSIBLE. /PAPAVERINE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 735


