



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
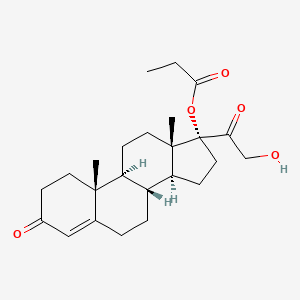

1. Cb-03-01
2. Clascoterone
3. Winlevi
1. Clascoterone
2. 19608-29-8
3. Cb-03-01
4. 17 Alpha-propionate
5. 17alpha-propionate
6. Winlevi
7. Cortexolone 17 Alpha-propionate
8. Clascoterone [usan]
9. Xn7mm8xg2m
10. Pregn-4-ene-3,20-dione, 21-hydroxy-17-(1-oxopropoxy)-
11. Cortexolone 17.alpha.-propionate
12. Cortodoxone 17.alpha.-propionate
13. Clascoterone (usan)
14. Pregn-4-ene-3,20-dione, 17,21-dihydroxy-, 17-propionate
15. (8r,9s,10r,13s,14s,17r)-17-(2-hydroxyacetyl)-10,13-dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl Propionate
16. [(8r,9s,10r,13s,14s,17r)-17-(2-hydroxyacetyl)-10,13-dimethyl-3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthren-17-yl] Propanoate
17. Breezula
18. Unii-xn7mm8xg2m
19. Cortodoxone 17alpha-propionate
20. 17
21. A-propionate
22. Clascoterone [mi]
23. Clascoterone [inn]
24. Cortexolone 17a-propionate
25. Clascoterone [who-dd]
26. Schembl1231152
27. Chembl3590187
28. Gtpl11215
29. Dtxsid10471883
30. Clascoterone [orange Book]
31. Bcp02565
32. Ex-a1953
33. Zinc6716459
34. 11-deoxycortisol 17alpha-propionate
35. S6896
36. Akos030526694
37. At22231
38. Bcp9000062
39. Cb-0301
40. Cs-1151
41. Db12499
42. 17alpha-(propionyloxy)deoxycorticosterone
43. Ac-31957
44. Hy-13331
45. Clascoterone; 19608-29-8; Cb-03-1
46. D11451
47. A854304
48. Q27293917
49. 21-hydroxy-3,20-dioxopregn-4-en-17-yl Propanoate
50. Cortexolone 17alpha-propionate;(8r,9s,10r,13s,14s,17r)-17-(2-hydroxyacetyl)-10,13-dimethyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl Propionate;cortexolone 17 Alpha-propionate
| Molecular Weight | 402.5 g/mol |
|---|---|
| Molecular Formula | C24H34O5 |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 402.24062418 g/mol |
| Monoisotopic Mass | 402.24062418 g/mol |
| Topological Polar Surface Area | 80.7 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 769 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Clascoterone is indicated for the topical treatment of acne vulgaris in patients 12 years of age and older.
Clascoterone exerts anti-androgenic effects by working as an antagonist at androgen receptors (ARs) expressed throughout the skin, including sebaceous glands, sebocytes, and dermal papilla cells. Clascoterone blocks the effects of testosterone and dihydrotestosterone (DHT), which are androgens that bind to the ARs and contribute to the development of androgen-dependent conditions such as acne and alopecia. _In vitro_, the antiandrogenic effects of clascoterone in human primary sebocytes occurred in a dose-dependent manner. Clascoterone mediates selective topical activity by mainly targeting androgen receptors at the site of application. It has limited systemic effects. In clinical trials, HPA axis suppression was observed as a 30-minute post-stimulation serum cortisol level of 18 mcg/dL in 5% of adult subjects and 9% of adolescent subjects with acne vulgaris following two weeks of topical treatment of clascoterone. HPA axis function returned to normal following the discontinuation of drug treatment.
D - Dermatologicals
D10 - Anti-acne preparations
D10A - Anti-acne preparations for topical use
D10AX - Other anti-acne preparations for topical use
D10AX06 - Clascoterone
Absorption
Upon topical application, clascoteronet permeates the skin to the dermal levels with minimal systemic absorption. In clinical trials, adult subjects with moderate to severe facial acne vulgaris received twice-daily topical application of six grams of clascoterone. The steady-state concentrations of the drug were reached within five days. Following two weeks, the mean SD Cmax was 4.5 2.9 ng/mL and the mean SD area under the plasma concentration-time over the dosing interval (AUC) was 37.1 22.3 h*ng/mL. The mean SD average plasma concentration (Cavg) was 3.1 1.9 ng/mL.
Route of Elimination
Excretion of clascoterone has not been fully characterized in humans. Upon topical application, clascoterone is quickly hydrolyzed in the epidermis.
Volume of Distribution
There is no information available on the volume of distribution.
Clearance
There is limited information on clearance of clascoterone.
According to _in vitro_ and clinical studies, the main possible primary metabolite of clascoterone is cortexolone, which is an inactive metabolite. The plasma concentrations of cortexolone were generally below or near the lower limit of quantitation (0.5 ng/mL). Although clascoterone penetrates the skin, the systemic activity of the drug is limited due to rapid hydrolysis of clascoterone into the inactive metabolite by skin and plasma esterases, namely carboxylesterase.
There is limited information on the half life of clascoterone.
Acne is a multifactorial skin condition characterized by excess sebum production, epithelial hyperkeratinization, proliferation of the skin commensal bacteria, and inflammation. Circulating and locally synthesized natural ligands, testosterone and dihydrotestosterone (DHT), serve as causative factors in both males and females. Upon binding of DHT, the DHT-androgen receptor complex dimerizes and translocates to the nucleus where it promotes the transcription of genes involved in acne pathogenesis, including proliferation and differentiation of sebocytes, excess sebum production, and inflammatory cytokine production. Clascoterone is a potent antagonist at ARs and competes for androgens in binding to the receptor, thereby inhibiting downstream signalling of ARs that promote acne. Androgenetic alopecia is also an androgen-dependent and highly genetic condition. Dihydrotestosterone (DHT) binds to ARs expressed on dermal papilla cells (DPC) in the scalp to induce AR-mediated transcription of genes that contribute to androgenic alopecia. By blocking the interaction between DHT and aARs, clascoterone inhibits AR-regulated transcription and DHT-induced IL-6 synthesis.


