



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0

1. Colimycin
2. Colisticin
3. Colistin
4. Colistin Sulfate
5. Coly-mycin
6. Sulfate, Colistin
7. Totazina
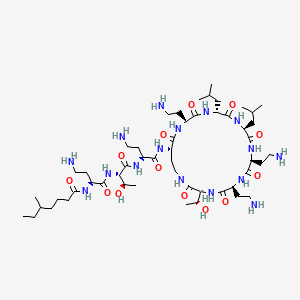
1. Colistin
2. 1066-17-7
3. N-[(2s)-4-amino-1-[[(2s,3r)-1-[[(2s)-4-amino-1-oxo-1-[[(3s,6s,9s,12s,15r,18s,21s)-6,9,18-tris(2-aminoethyl)-3-[(1r)-1-hydroxyethyl]-12,15-bis(2-methylpropyl)-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptazacyclotricos-21-yl]amino]butan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-1-oxobutan-2-yl]-5-methylheptanamide
4. Colobreathe
5. Promixin
6. Colistin,(s)
7. Chembl499783
8. Schembl1979092
9. Gtpl10794
10. Colistin Sulfate, >19000 Iu/mg
11. Ab01566924_01
12. 066c177
13. Q418946
14. Sr-01000872582
15. Sr-01000872582-1
16. N-[(1s)-3-amino-1-[[(1s,2r)-1-[[(1s)-3-amino-1-[[(3s,6s,9s,12s,15r,18s,21s)-6,9,18-tris(2-aminoethyl)-3-[(1r)-1-hydroxyethyl]-12,15-diisobutyl-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptazacyclotricos-21-yl]carbamoyl]propyl]carbamoyl]-2-hydroxy-propyl]carbamoyl]propyl]-5-methyl-heptanamide
17. N-[(1s)-3-amino-1-{[(1s,2r)-1-{[(1s)-3-amino-1-{[(3s,6s,9s,12s,15r,18s,21s)-6,9,18-tris(2-aminoethyl)-3-[(1r)-1-hydroxyethyl]-12,15-bis(2-methylpropyl)-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptaazacyclotricosan-21-yl]carbamoyl}propyl]carbamoyl}-2-hydroxypropyl]carbamoyl}propyl]-5-methylheptanamide
| Molecular Weight | 1155.4 g/mol |
|---|---|
| Molecular Formula | C52H98N16O13 |
| XLogP3 | -3.3 |
| Hydrogen Bond Donor Count | 18 |
| Hydrogen Bond Acceptor Count | 18 |
| Rotatable Bond Count | 28 |
| Exact Mass | 1154.74992724 g/mol |
| Monoisotopic Mass | 1154.74992724 g/mol |
| Topological Polar Surface Area | 491 Ų |
| Heavy Atom Count | 81 |
| Formal Charge | 0 |
| Complexity | 2050 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 12 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antibiotics, Peptide
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
THERAPEUTIC INDICATIONS FOR COLISTIN ARE ESSENTIALLY SAME AS THOSE FOR POLYMYXIN B. INFECTIONS OF CERTAIN TYPES CAUSED BY PSEUD AERUGINOSA ARE ESPECIALLY SUSCEPTIBLE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1233
PRIMARY USE OF POLYMYXIN B IS FOR TREATMENT OF INFECTIONS CAUSED BY GRAM-NEGATIVE BACTERIA, ESPECIALLY PSEUDOMONAS. ...IS EFFECTIVE IN TREATMENT OF URINARY TRACT INFECTIONS CAUSED BY PSEUDOMONAS OR OTHER GRAM-NEGATIVE BACILLI RESISTANT TO OTHER ANTIMICROBIAL AGENTS... /POLYMYXIN B/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1232
/POLYMYXIN B & COLISTIMETHATE SODIUM/...RECOMMENDED FOR TREATING PERITONITIS & PNEUMONIA, BUT SOME AUTHORITIES QUESTION THEIR EFFECTIVENESS FOR THESE PURPOSES. /COLISTIMETHATE SODIUM/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 734
For more Therapeutic Uses (Complete) data for COLISTIN (11 total), please visit the HSDB record page.
ADVERSE REACTIONS TO COLISTIMETHATE HAVE BEEN NOTED IN 20% OF PT GIVEN THE DRUG; THEY ARE GENERALLY REVERSIBLE... /COLISTIMETHATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1233
COLISTIMETHATE SODIUM SHOULD NOT BE ADMIN INTRATHECALLY. /COLISTIMETHATE SODIUM/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 734
...NOT INDICATED FOR INFECTIONS CAUSED BY PROTEUS OR NEISSERIA SPECIES. /SODIUM COLISTIMETHATE/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 394
POSSIBLE EMBRYOTOXIC & TERATOGENIC EFFECTS HAVE BEEN REPORTED WHEN COLISTIMETHATE SODIUM WAS ADMINISTERED TO PREGNANT RABBITS. /NA COLISTIMETHATE/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 395
For more Drug Warnings (Complete) data for COLISTIN (13 total), please visit the HSDB record page.
Colobreathe is indicated for the management of chronic pulmonary infections due to Pseudomonas aeruginosa in patients with cystic fibrosis (CF) aged six years and older.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01XB01
A - Alimentary tract and metabolism
A07 - Antidiarrheals, intestinal antiinflammatory/antiinfective agents
A07A - Intestinal antiinfectives
A07AA - Antibiotics
A07AA10 - Colistin
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01X - Other antibacterials
J01XB - Polymyxins
J01XB01 - Colistin
/COLISTIMETHATE SODIUM/...IS EXCRETED IN DOG URINE TO MUCH GREATER EXTENT THAN SIMPLE SULFATE FORM, & THEORETICALLY SHOULD BE BETTER IN URINARY INFECTIONS.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 130
DRUG PASSES FROM MATERNAL TO FETAL CIRCULATION. PREMATURE INFANTS INJECTED WITH 1 MG/KG DO NOT DEVELOP EFFECTIVE PLASMA CONCN OF ANTIBIOTIC; WITH DOSE OF 2 MG/KG, PEAK VALUE OF ABOUT 5 UG/ML IS REACHED IN 30 MIN. /COLISTIMETHATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1233
PLASMA CONCN ARE HIGHER IN PERSONS WITH RENAL INSUFFICIENCY & ARE RELATED TO DEGREE OF RENAL DYSFUNCTION. COLISTIMETHATE IS EXCRETED MAINLY BY GLOMERULAR FILTRATION. URINE CONCN EXCEED 200 UG/ML DURING FIRST 2 HR AFTER USUAL IM DOSE. /COLISTIMETHATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1233
DRUG IS EXCRETED MORE RAPIDLY IN CHILDREN THAN IN ADULTS. COLISTIMETHATE DOES NOT GAIN ACCESS TO CEREBROSPINAL FLUID, EVEN WHEN MENINGES ARE INFLAMED. /COLISTIMETHATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1233
For more Absorption, Distribution and Excretion (Complete) data for COLISTIN (8 total), please visit the HSDB record page.
...hydrolyzed in vivo to colistin and possibly other metabolites with fewer substituted amino groups...
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 394
AFTER IV INJECTION RABBITS, 75% DOSE EXCRETED IN URINE UNCHANGED. SMALL AMT FOUND IN BILE. COLISTIN-N-GLUCURONIDE FOUND IN URINE (1.7% OF DOSE) & BILE (6.7% OF DOSE). /SODIUM COLISTIN METHANE SULFONATE/
ABE ET AL; CHEMOTHERAPY (TOKYO) 24(8) 1592-1596 (1976)
IM INJECTION OF 150 MG OF COLISTIMETHATE IN ADULTS PRODUCE PEAK PLASMA CONCN OF 6 UG/ML @ 2 HR; THIS DECLINES WITH HALF-TIME OF 2 HR. SAME QUANTITY GIVEN IV YIELDS MAXIMAL PLASMA CONCN OF 18 UG/ML; THIS FALLS TO ABOUT 0.4 UG/ML @ 12 HR. /COLISTIMETHATE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1233
POLYMYXIN B IS SURFACE-ACTIVE AGENT... CONTAINING LIPOPHILIC & LIPOPHOBIC GROUPS SEPARATED WITHIN MOLECULE. /POLYMYXIN B/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1144
PERMEABILITY OF THE BACTERIAL MEMBRANE CHANGES IMMEDIATELY ON CONTACT WITH DRUG. SENSITIVITY TO POLYMYXIN B APPARENTLY IS RELATED TO THE PHOSPHOLIPID CONTENT OF THE CELL WALL-MEMBRANE COMPLEX. /POLYMYXIN B/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1144
Colistin acts like a cationic detergent and binds to and damages the bacterial cytoplasmic membrane of susceptible bacteria. Damage to the bacterial cytoplasmic membrane alters the osmotic barrier of the membrane and causes leakage of essential intracellular metabolites and nucleosides.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 97. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1997 (Plus Supplements)., p. 394


