
Synopsis
Synopsis
0
KDMF
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
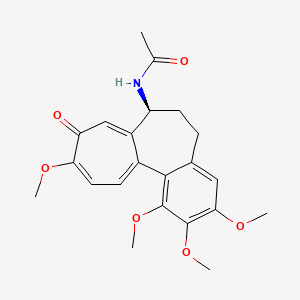

1. Colchicine, (+-)-isomer
2. Colchicine, (r)-isomer
1. 64-86-8
2. Colchisol
3. Colchineos
4. Colchicin
5. Colcin
6. Colchicina
7. Colchicinum
8. Condylon
9. Colsaloid
10. Colcrys
11. 7alphah-colchicine
12. Kolkicin
13. Goutnil
14. (s)-colchicine
15. (-)-colchicine
16. Mitigare
17. Spindle Poison
18. Nsc 757
19. N-[(7s)-1,2,3,10-tetramethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl]acetamide
20. N-acetyl Trimethylcolchicinic Acid Methylether
21. Nsc757
22. N-[(7s)-1,2,3,10-tetramethoxy-9-oxo-6,7-dihydro-5h-benzo[a]heptalen-7-yl]acetamide
23. Nsc-757
24. (s)-n-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[a]heptalen-7-yl)acetamide
25. Mfcd00078484
26. Chembl107
27. Sml2y3j35t
28. Chebi:27882
29. Colchicine [jan]
30. Colchicin [german]
31. Ncgc00025125-07
32. Colchysat
33. Colchicina [italian]
34. 7-alpha-h-colchicine
35. Dsstox_cid_4845
36. Dsstox_rid_77551
37. Dsstox_gsid_24845
38. Colstat
39. N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)acetamide
40. Acetamide, N-((7s)-5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)-
41. Acetamide, N-[(7s)-5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[a]heptalen-7-yl]-
42. Colchicine (tn)
43. N-[(7s)-1,2,3,10-tetramethoxy-9-oxo-6,7-dihydro-5h-benzo[d]heptalen-7-yl]ethanamide
44. 30512-31-3
45. Loc
46. Smr000058323
47. Ccris 691
48. (s)-colchicine >95%
49. 7.alpha.h-colchicine
50. Hsdb 3044
51. Acetamide, N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)-, (s)-
52. Colchicine [usp:jan]
53. Sr-01000075794
54. Sr-01000597576
55. Einecs 200-598-5
56. Unii-sml2y3j35t
57. Colchcine
58. Ai3-31149
59. (s)-colchicina
60. 4lzr
61. (s)-colchicin
62. Acetamide,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(.alpha.)heptalen-7-yl)-
63. Acetamide,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[.alpha.]heptalen-7-yl)-
64. Mpc-004
65. N-((7s)-5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)- Acetamide
66. Cas-64-86-8
67. (s)-n-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)acetamide
68. Prestwick_695
69. Gloperba
70. Spectrum_000842
71. Tocris-1364
72. 4o2b
73. Colchicine [mi]
74. Prestwick0_000363
75. Prestwick1_000363
76. Prestwick2_000363
77. Prestwick3_000363
78. Spectrum2_000075
79. Spectrum3_000362
80. Spectrum4_000298
81. Spectrum5_000787
82. Colchcine [vandf]
83. Colchicine [hsdb]
84. Colchicinum [hpus]
85. Colchicine (jp17/usp)
86. Upcmld-dp065
87. Benzo(a)heptalen-9(5h)-one, 7-acetamido-6,7-dihydro-1,2,3,10-tetramethoxy-
88. C 9754
89. Ec 200-598-5
90. Colchicine [mart.]
91. Schembl8469
92. Colchicine [usp-rs]
93. Colchicine [who-dd]
94. Colchicine [who-ip]
95. N-[(7s)-5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[a]heptalen-7-yl]acetamide
96. Lopac0_000310
97. Methoxylated Analogue Of Xd1
98. Bspbio_000485
99. Bspbio_002083
100. Kbiogr_000856
101. Kbioss_001322
102. Colchicine (contains 5% Ethyl Acetate At Maximum)
103. Mls001055448
104. Mls001304089
105. Mls002153786
106. Divk1c_000753
107. Spectrum1500205
108. Spbio_000289
109. Spbio_002406
110. Bpbio1_000535
111. Gtpl2367
112. Megxp0_001879
113. Colchicine [ep Impurity]
114. Colchicine [orange Book]
115. Dtxsid5024845
116. Upcmld-dp065:001
117. Acon1_000353
118. Hms502f15
119. Kbio1_000753
120. Kbio2_001322
121. Kbio2_003890
122. Kbio2_006458
123. Kbio3_001303
124. Colchicine [ep Monograph]
125. Ninds_000753
126. Colchicine [usp Monograph]
127. Hms1569i07
128. Hms1920a08
129. Hms2091g16
130. Hms2096i07
131. Hms2231c05
132. Hms3260n22
133. Hms3713i07
134. Pharmakon1600-01500205
135. Zinc621853
136. Colchicinum [who-ip Latin]
137. Colchicine, >=96.0% (hplc)
138. Mitigare Component Colchicine
139. Proben-c Component Colchicine
140. Tox21_110947
141. Tox21_201547
142. Tox21_300582
143. Tox21_500310
144. Bdbm50014846
145. Ccg-39910
146. Nsc756702
147. Nsc813203
148. S2284
149. Colbenemid Component Colchicine
150. Akos001582887
151. Colchicine, >=95% (hplc), Powder
152. Tox21_110947_1
153. Colchicine Component Of Mitigare
154. Cs-1141
155. Db08117
156. Lp00310
157. Nsc-756702
158. Nsc-813203
159. Sdccgmls-0066633.p001
160. Sdccgsbi-0050298.p006
161. Colchicine Component Of Proben-c
162. Idi1_000753
163. N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo[.alpha.]heptalen-7-yl)-acetamide
164. Colchicine Component Of Colbenemid
165. Ncgc00025125-01
166. Ncgc00025125-02
167. Ncgc00025125-03
168. Ncgc00025125-04
169. Ncgc00025125-05
170. Ncgc00025125-06
171. Ncgc00025125-08
172. Ncgc00025125-09
173. Ncgc00025125-10
174. Ncgc00025125-11
175. Ncgc00025125-12
176. Ncgc00025125-13
177. Ncgc00025125-14
178. Ncgc00025125-15
179. Ncgc00025125-18
180. Ncgc00025125-20
181. Ncgc00025125-33
182. Ncgc00169157-01
183. Ncgc00169157-02
184. Ncgc00169157-03
185. Ncgc00254359-01
186. Ncgc00259096-01
187. Ncgc00260995-01
188. As-13686
189. Col-probenecid Component Colchicine
190. Hy-16569
191. Nci60_041659
192. Sbi-0050298.p004
193. Colchicine Component Of Col-probenecid
194. Eu-0100310
195. Ft-0603187
196. Mls001055448-02
197. N1721
198. C-7100
199. C07592
200. D00570
201. M01514
202. Binds To Tubulin; Inhibits Microtubular Assembly
203. 078c484
204. Sr-01000075794-1
205. Sr-01000075794-3
206. Sr-01000075794-6
207. Sr-01000075794-7
208. Sr-01000597576-1
209. Sr-01000597576-3
210. Brd-k00259736-001-06-5
211. Brd-k00259736-001-10-7
212. Wln: L B677 Mv&t&j Co1 Do1 Eo1 Jmv1 No1
213. Benzo[a]heptalen-9(5h)-one,7-dihydro-1,2,3,10-tetramethoxy-
214. Colchicine, (european Pharmacopoeia (ep) Reference Standard)
215. Colchicine, United States Pharmacopeia (usp) Reference Standard
216. Colchicine, Bioreagent, Plant Cell Culture Tested, >=95% (hplc)
217. Colchicine, Pharmaceutical Secondary Standard; Certified Reference Material
218. Colchicine For System Suitability, European Pharmacopoeia (ep) Reference Standard
219. N-[(7s)-1,2,3,10-tetramethoxy-9-oxo-6,7-dihydro-5h-benzo[d]heptalen-7-yl]acetamide
220. (1e)-n-[(7s)-1,2,3,10-tetramethoxy-9-oxo-5,6,7,9-tetrahydrobenzo[a]heptalen-7-yl] Ethanimidic Acid
221. Acetamide, N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo(a)heptalen-7-yl)-,(s)-
| Molecular Weight | 399.4 g/mol |
|---|---|
| Molecular Formula | C22H25NO6 |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 399.16818752 g/mol |
| Monoisotopic Mass | 399.16818752 g/mol |
| Topological Polar Surface Area | 83.1 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 740 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Colcrys |
| PubMed Health | Colchicine (By mouth) |
| Drug Classes | Antigout |
| Drug Label | Colchicine is an alkaloid chemically described as (S)N- (5,6,7,9-tetrahydro- 1,2,3, 10-tetramethoxy-9-oxobenzo [alpha] heptalen-7-yl) acetamide with a molecular formula of C22H25NO6 and a molecular weight of 399.4. The structural formula of colchicin... |
| Active Ingredient | Colchicine |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 0.6mg |
| Market Status | Prescription |
| Company | Ar Holding; Takeda Pharms Usa |
| 2 of 2 | |
|---|---|
| Drug Name | Colcrys |
| PubMed Health | Colchicine (By mouth) |
| Drug Classes | Antigout |
| Drug Label | Colchicine is an alkaloid chemically described as (S)N- (5,6,7,9-tetrahydro- 1,2,3, 10-tetramethoxy-9-oxobenzo [alpha] heptalen-7-yl) acetamide with a molecular formula of C22H25NO6 and a molecular weight of 399.4. The structural formula of colchicin... |
| Active Ingredient | Colchicine |
| Dosage Form | Tablet |
| Route | oral; Oral |
| Strength | 0.6mg |
| Market Status | Prescription |
| Company | Ar Holding; Takeda Pharms Usa |
Gout Suppressants
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Colchicine also is used in the prophylactic treatment of recurrent gouty arthritis. Colchicine has no effect on plasma concentrations or urinary excretion of uric acid; therefore, concomitant administration of allopurinol or a uricosuric agent (e.g., probenecid, sulfinpyrazone) is necessary to decrease serum urate concentrations. Prophylactic doses of colchicine should be administered before the initiation of allopurinol or uricosuric therapy because sudden changes in serum urate concentrations may precipitate acute gout attacks. After the serum urate concentration has been reduced to the desired level and acute gout attacks have not occurred for 3-6 months (some clinicians suggest 1-12 months), colchicine may be discontinued and the patient may be treated with urate lowering agents alone. Colchicine is frequently used in combination with probenecid to facilitate prophylactic therapy in patients with chronic gouty arthritis. The usefulness of the commercially available fixed-dosage preparation is limited, however, because the colchicine present exceeds the amount required by most patients. /Use Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3691-2
Colchicine is used to relieve attacks of acute gouty arthritis. Nonsteroidal anti-inflammatory agents (NSAIAs) (e.g., indomethacin, ibuprofen, naproxen, sulindac, piroxicam, ketoprofen) are as effective as, and better tolerated than, usual dosages of colchicine for short-term use in relieving acute attacks of gouty arthritis. Corticosteroids also are used to relieve acute attacks of gouty arthritis. Colchicine is considered a second-line agent; colchicine may be used for the treatment of acute gouty arthritis in patients who have not responded to or who cannot tolerate recommended therapies (i.e., NSAIAs, corticosteroids). /Use Included in US product label/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3691
96 patients aged 15 yr or more with complete or incomplete Behcet's disease, whose visual acuity was 20/40 or less, and who had experienced at least 2 episodes of ocular attack during the 16 wk before the study were selected. 47 patients received cyclosporin (10 mg/kg) and 49 colchicine (1 mg/kg) daily for 16 wk. The frequency of ocular attack was reduced more in the cyclosporin group than in the colchicine group (p < 0.001). The severity of ocular attacks was also less severe after cyclosporin than after colchicine (p < 0.001). Colchicine alleviated oral aphthous ulcer in 10 patients (20%). Dermal lesions were alleviated in 15% of the colchicine group. Clinical symptoms were improved in 33% for the colchicine group, and 10 cases were aggravated. OKT4/OKT8 ratios were 1.44 in the colchicine group before the study and 1.46 after treatment. Frequently observed side effects of colchicine were hirsutism (2 patients) and renal dysfunction (2 patients). Treatment was stopped because of hepatic dysfunction in 2 colchicine cases.
PMID:2566048 Masuda K et al; Lancet 1 (8647): 1093-6 (1989)
For more Therapeutic Uses (Complete) data for COLCHICINE (11 total), please visit the HSDB record page.
Colchicine injection has been available in the US since the 1950s and has been used for the treatment of acute attacks of gout. Colchicine injection preparations that have been commercially available have not been approved by the US Food and Drug Administration (FDA). Serious adverse events, some fatal, have been reported in patients receiving colchicine injection. Because of the potentially serious health risks associated with unapproved colchicine injection, FDA announced on February 8, 2008, that it would take enforcement action (e.g., seizure, injunction, other judicial proceeding) against all firms, including compounding pharmacies, attempting to manufacture, ship, or deliver colchicine injection. FDA will implement enforcement action against all firms attempting to manufacture or ship colchicine injection products that do not have a National Drug Code (NDC) number on or after February 8, 2008. For colchicine injection products with an NDC number, FDA will take enforcement action against all firms attempting to manufacture such products on or after March 10, 2008, and against firms that ship such products on or after August 6, 2008.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3692
Myelosuppression, leukopenia, granulocytopenia, thrombocytopenia, pancytopenia, and aplastic anemia with colchicine used in therapeutic doses have been reported.
US Natl Inst Health; DailyMed. Current Medication Information for Colcrys - Colchicine, USP (September 2009). Available from, as of September 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15266
Colchicine-induced neuromuscular toxicity and rhabdomyolysis have been reported with chronic treatment in therapeutic doses. Patients with renal dysfunction and elderly patients, even those with normal renal and hepatic function, are at increased risk.
US Natl Inst Health; DailyMed. Current Medication Information for Colcrys - Colchicine, USP (September 2009). Available from, as of September 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15266
The most common adverse reaction is diarrhea (23%). Pharyngolaryngeal pain was seen in 3% of patients treated for gout flares. Gastrointestinal tract adverse effects are the most frequent side effects in patients initiating colchicine, usually presenting within 24 hours, and occurring in up to 20% of patients given therapeutic doses. Typical symptoms include cramping, nausea, diarrhea, abdominal pain, and vomiting. These events should be viewed as dose-limiting if severe as they can herald the onset of more significant toxicity.
US Natl Inst Health; DailyMed. Current Medication Information for Colcrys - Colchicine, USP (September 2009). Available from, as of September 29, 2009: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15266
For more Drug Warnings (Complete) data for COLCHICINE (13 total), please visit the HSDB record page.
The lethal dose in humans has been estimated to be 65 mg.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3693
Prevention of cardiovascular events
Gout Suppressants
Agents that increase uric acid excretion by the kidney (URICOSURIC AGENTS), decrease uric acid production (antihyperuricemics), or alleviate the pain and inflammation of acute attacks of gout. (See all compounds classified as Gout Suppressants.)
Tubulin Modulators
Agents that interact with TUBULIN to inhibit or promote polymerization of MICROTUBULES. (See all compounds classified as Tubulin Modulators.)
M04AC01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M - Musculo-skeletal system
M04 - Antigout preparations
M04A - Antigout preparations
M04AC - Preparations with no effect on uric acid metabolism
M04AC01 - Colchicine
The absorption of colchicine is rapid but variable. Peak plasma concentrations occur 0.5 to 2 hours after dosing. In plasma, 50% of colchicine is protein-bound. There is significant enterohepatic circulation. The exact metabolism of colchicine is unknown but seems to involve deacetylation by the liver. Only 10% to 20% is excreted in the urine, although this increases in patients with liver disease. The kidney, liver, and spleen also contain high concentrations of colchicine, but it apparently is largely excluded from heart, skeletal muscle, and brain. The plasma half-life of colchicine is approximately 9 hours, but it can be detected in leukocytes and in the urine for at least 9 days after a single intravenous dose.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 707
... Two cases involving suicide by the ingestion of medications marketed in France /is reported/. In case 1, only heart blood was taken after body external examination. In case 2 an autopsy was performed and heart blood, urine, gastric contents and bile were taken for toxicological analysis. Colchicine was assayed in biological specimens by an HPLC-DAD method, after extraction by dichloromethane at pH 8, adding prazepam as internal standard (IS). Analyses were performed on a Symetry C-8 column. Mobile phase was a gradient of acetonitrile/pH 3.8 phosphate buffer. Colchicine is eluted at 13.1 min and the method is linear for blood, urine and bile over the range 4-1000 ng/mL. LOQ is 4 ng/mL. The concentrations of colchicine detected are: case 1: heart blood 13 ng/mL; case 2: heart blood 66 ng/mL, urine 500 ng/mL, gastric content 12 ng/mL, bile 5632 ng/mL. Our findings are in the range of lethal concentrations previously described, but there is no correlation with the amount of ingested drug. Even after massive overdose, it could be impossible to detect colchicine in blood, and as there is a widespread enterohepatic recirculation before excretion in bile and feces, bile is the target sample to analyse. We conclude in both cases that the cause of death was suicide with colchicine. It appears very important to perform an autopsy in order to obtain bile, urine, heart blood and femoral blood.
PMID:15240048 Deveaux M et al; Forensic Sci Int 143 (2-3): 219-22 (2004).
After oral administration plasma concentrations reach a peak within 0.5 to 2 hours and afterwards decrease rapidly within 2 hours. The plasma half-life is 60 minutes. Colchicine may remain in tissues for as long as 10 days.
IPCS; Poisons Information Monograph (PIM) 141: Colchicine. (October 1995). Available from, as of August 25, 2009: https://www.inchem.org/documents/pims/pharm/colchic.htm
Information was available on urinary excretion in 5 cases. Concentrations in urine are 10 to 80 fold higher than those in plasma. Four to 25 per cent of the dose ingested was excreted in urine over three to ten days. Excretion was specially high during the first 24 hours following ingestion. Colchicine is eliminated in urine up to the tenth day.
IPCS; Poisons Information Monograph (PIM) 141: Colchicine. (October 1995). Available from, as of August 25, 2009: https://www.inchem.org/documents/pims/pharm/colchic.htm
For more Absorption, Distribution and Excretion (Complete) data for COLCHICINE (12 total), please visit the HSDB record page.
Colchicine undergoes some hepatic metabolism. Colchicine is partially deacetylated in the liver. Large amounts of colchicine and of its metabolites undergo enterohepatic circulation. This may explain the occurrence of a second plasma peak concentration observed 5 to 6 hours after ingestion.
IPCS; Poisons Information Monograph (PIM) 141: Colchicine. (October 1995). Available from, as of August 25, 2009: https://www.inchem.org/documents/pims/pharm/colchic.htm
Three novel conjugation metabolites of colchicine were identified in rat bile facilitated by enhanced on-line liquid chromatography-accurate radioisotope counting. The known 2- and 3-demethylcolchicines (DMCs) underwent O-sulfate conjugation in addition to the previously described O-glucuronidation. 2-DMC was preferably O-glucuronidated, whereas 3-DMC predominantly yielded O-sulfation conjugates, indicating phase II conjugation regiopreferences. Moreover, M1 was identified as a novel glutathione conjugate and a possible biotransformation pathway for its formation was proposed. The known 2-DMC (M6), 3-DMC (M7), 2-DMC glucuronide (M4), and novel 3-DMC sulfate (M3) were confirmed as the major metabolites. ...
PMID:18227142 Xu L et al; Drug Metab Dispos 36 (4): 731-9 (2008).
Following IV administration of a single therapeutic dose (as of August 2008, IV preparations are no longer commercially available in the US), colchicine is rapidly removed from the plasma; plasma half-life is about 20 minutes. The drug has a half-life of about 60 hours in leukocytes.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 3694
The elimination half life is variable, ranging from 4.4 hours in normal patients to 30 hours or more in elderly patients. A therapeutic dose produced a half life of 18.8 hours in patients with renal dysfunction.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1033
After a single 2 mg intravenous dose the average plasma half life is 20 minutes. Plasma half-life is increased in severe renal disease (40 min) and decreased in severe hepatic disease (9 min).
IPCS; Poisons Information Monograph (PIM) 141: Colchicine. (October 1995). Available from, as of August 25, 2009: https://www.inchem.org/documents/pims/pharm/colchic.htm
... HUVEC cells were exposed to various concentrations of colchicine and were harvested at different time points. Ribonucleic acid was extracted, amplified, reverse transcribed and hybridized to complementary deoxyribonucleic acid microarrrays containing more than 40,000 probes to human expressed sequence tags. This approach enabled us to have a global look at the transcriptional response induced by colchicine treatment. Colchicine changed the expression of many genes in HUVEC cells following exposure to a concentration of 100 ng/ml or higher. Following short exposure (30 or 120 min), colchicine affected genes known to be involved in the cell cycle and its regulation. However, change in expression of genes involved in neutrophil migration or other inflammatory processes were observed mainly after 12 to 24 hr. The anti-inflammatory effect of colchicine may be mediated not only through direct interaction with microtubules but also through changes at the transcriptional level. This latter effect apparently requires a higher concentration and a longer time to occur.
PMID:16188942 Ben-Chetrit E et al; Rheumatology (Oxford) 45 (3):274-82 (2006).
Colchicine, long used to treat gout, arrests microtubule assembly and inhibits many cellular functions. At micromolar concentrations, it suppresses monosodium urate crystal-induced NACHT-LRR-PYD-containing protein-3 (NALP3) inflammasome-driven caspase-1 activation, IL-1beta processing and release, and L-selectin expression on neutrophils. At nanomolar concentrations, colchicine blocks the release of a crystal-derived chemotactic factor from neutrophil lysosomes, blocks neutrophil adhesion to endothelium by modulating the distribution of adhesion molecules on the endothelial cells, and inhibits monosodium urate crystal-induced production of superoxide anions from neutrophils. Cyto-chrome P450 3A4, the multidrug transporter P-glycoprotein, and the drugs that bind these proteins influence its pharmacokinetics and pharmacodynamics. Trial evidence supports its efficacy in acute gout and in preventing gout flares, but it has narrow therapeutic index, and overdosage is associated with gastrointestinal, hepatic, renal, neuromuscular, and cerebral toxicity; bone marrow damage; and high mortality.
PMID:18638431 Nuki G; Curr Rheumatol Rep 10 (3): 218-27 (2008).
The actions of colchicine were examined with the two-electrode voltage-clamp technique and radioligand binding assays in mouse and human 5-hydroxytryptamine(3A) receptors (5-HT(3A)Rs) expressed in Xenopus laevis oocytes. Colchicine inhibited 5-hydroxytryptamine (5-HT)-evoked currents in oocytes expressing mouse 5-HT(3A)Rs, with an IC(50) of 59.5 +/- 3 uM. In contrast to the mouse receptor, coapplication of colchicine with 5-HT (<1 uM) strongly enhanced 5-HT-evoked currents in oocytes expressing human 5-HT(3A)Rs. Colchicine applied alone did not induce a detectable current. In the presence of 0.5 microM 5-HT, the potentiation was concentration-dependent and reached the maximum (approximately 100%) when 750 microM colchicine was applied. However, colchicine-dependent inhibition can be observed at 5-HT concentrations > 1 uM. In oocyte membranes expressing mouse or human receptors, binding studies with colchicine (25 nM-1 mM) revealed no displacement of 1-methyl-N-((1R,3r,5S)-9-methyl-9 azabicyclo [3.3.1]nonan-3yl)-1H-indazole-3 carboxamide ([(3)H]BRL-43694), suggesting that actions of colchicine do not occur at the ligand binding domain. Functional effects of colchicine on both receptors occurred in the absence of preincubation and after cold temperature incubation, suggesting that the microtubule-depolymerizing effects of colchicine play no role in modulation of receptor function. Studies with interspecies chimeric receptors demonstrated that the distal one third of the N terminus is responsible for the bidirectional modulation by colchicine. Collectively, these results suggest that colchicine modulates receptor function through loops C and/or F through a gating mechanism.
PMID:19188483 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2672862 de Oliveira-Pierce AN et al; J Pharmacol Exp Ther 329 (2): 838-47 (2009).
Colchicine exerts a variety of pharmacological effects, but how these occur or how they relate to its activity in gout is not well understood. It has antimitotic effects, arresting cell division in GI by interfering with microtubule and spindle formation (an effect shared with vinca alkaloids). This effect is greatest on cells with rapid turnover (e.g., neutrophils and GI epithelium). Although somewhat controversial, colchicine may alter neutrophil motility in ex vivo assays. Colchicine also renders cell membranes more rigid and decreases the secretion of chemotactic factors by activated neutrophils.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 707
For more Mechanism of Action (Complete) data for COLCHICINE (8 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Regulatory Info : Prescription
Registration Country : Denmark
Brand Name : Colchicin \"Strides\"
Dosage Form : Tablet
Dosage Strength : 0.5mg
Packaging :
Approval Date : 19-09-2022
Application Number : 28106588021
Regulatory Info : Prescription
Registration Country : Denmark
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Approved
Registration Country : Sweden
Brand Name : Colchicine 2Care4
Dosage Form : Tablet
Dosage Strength : 500mcg
Packaging :
Approval Date : 21-03-2024
Application Number : 2.02E+13
Regulatory Info : Approved
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Withdrawn
Registration Country : Malta
Brand Name : Colchicine
Dosage Form : Film Coated Tablet
Dosage Strength : 1000MCG
Packaging :
Approval Date : 2018-06-18
Application Number :
Regulatory Info : Withdrawn
Registration Country : Malta

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Approved
Registration Country : Sweden
Brand Name : Colrefuz
Dosage Form : Tablet
Dosage Strength : 500mcg
Packaging :
Approval Date : 25-05-2023
Application Number : 2.02E+13
Regulatory Info : Approved
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Marketed
Registration Country : Norway
Brand Name : Colrefuz
Dosage Form : Tablet
Dosage Strength : 500mcg
Packaging :
Approval Date :
Application Number :
Regulatory Info : Marketed
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Deregistered
Registration Country : Sweden
Brand Name : Colrefuz
Dosage Form : Tablet
Dosage Strength : 500mcg
Packaging :
Approval Date : 12-09-2023
Application Number : 2.02E+13
Regulatory Info : Deregistered
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Suspended
Registration Country : Spain
Brand Name : Colchicine Ria
Dosage Form : Tablet
Dosage Strength : 1MG
Packaging :
Approval Date : 10-06-2020
Application Number : 84475
Regulatory Info : Suspended
Registration Country : Spain

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Authorized
Registration Country : Spain
Brand Name : Colchicine Seid
Dosage Form : Tablet
Dosage Strength : 0.5MG
Packaging :
Approval Date : 29-07-2014
Application Number : 78947
Regulatory Info : Authorized
Registration Country : Spain

Regulatory Info : Authorised
Registration Country : Malta
Brand Name : Colchicine Tiofarma
Dosage Form : Tablet
Dosage Strength : 0.5MG
Packaging :
Approval Date : 2022-04-25
Application Number :
Regulatory Info : Authorised
Registration Country : Malta

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Regulatory Info : Authorised
Registration Country : Malta
Brand Name : Colchicine
Dosage Form : Tablet
Dosage Strength : 500MCG
Packaging :
Approval Date : 2024-11-19
Application Number :
Regulatory Info : Authorised
Registration Country : Malta

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
85
PharmaCompass offers a list of Colchicine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Colchicine manufacturer or Colchicine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Colchicine manufacturer or Colchicine supplier.
PharmaCompass also assists you with knowing the Colchicine API Price utilized in the formulation of products. Colchicine API Price is not always fixed or binding as the Colchicine Price is obtained through a variety of data sources. The Colchicine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Colchicine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Colchicine, including repackagers and relabelers. The FDA regulates Colchicine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Colchicine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Colchicine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Colchicine supplier is an individual or a company that provides Colchicine active pharmaceutical ingredient (API) or Colchicine finished formulations upon request. The Colchicine suppliers may include Colchicine API manufacturers, exporters, distributors and traders.
click here to find a list of Colchicine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Colchicine DMF (Drug Master File) is a document detailing the whole manufacturing process of Colchicine active pharmaceutical ingredient (API) in detail. Different forms of Colchicine DMFs exist exist since differing nations have different regulations, such as Colchicine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Colchicine DMF submitted to regulatory agencies in the US is known as a USDMF. Colchicine USDMF includes data on Colchicine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Colchicine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Colchicine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Colchicine Drug Master File in Japan (Colchicine JDMF) empowers Colchicine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Colchicine JDMF during the approval evaluation for pharmaceutical products. At the time of Colchicine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Colchicine suppliers with JDMF on PharmaCompass.
A Colchicine CEP of the European Pharmacopoeia monograph is often referred to as a Colchicine Certificate of Suitability (COS). The purpose of a Colchicine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Colchicine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Colchicine to their clients by showing that a Colchicine CEP has been issued for it. The manufacturer submits a Colchicine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Colchicine CEP holder for the record. Additionally, the data presented in the Colchicine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Colchicine DMF.
A Colchicine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Colchicine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Colchicine suppliers with CEP (COS) on PharmaCompass.
A Colchicine written confirmation (Colchicine WC) is an official document issued by a regulatory agency to a Colchicine manufacturer, verifying that the manufacturing facility of a Colchicine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Colchicine APIs or Colchicine finished pharmaceutical products to another nation, regulatory agencies frequently require a Colchicine WC (written confirmation) as part of the regulatory process.
click here to find a list of Colchicine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Colchicine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Colchicine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Colchicine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Colchicine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Colchicine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Colchicine suppliers with NDC on PharmaCompass.
Colchicine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Colchicine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Colchicine GMP manufacturer or Colchicine GMP API supplier for your needs.
A Colchicine CoA (Certificate of Analysis) is a formal document that attests to Colchicine's compliance with Colchicine specifications and serves as a tool for batch-level quality control.
Colchicine CoA mostly includes findings from lab analyses of a specific batch. For each Colchicine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Colchicine may be tested according to a variety of international standards, such as European Pharmacopoeia (Colchicine EP), Colchicine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Colchicine USP).

