
Synopsis
Synopsis
0
VMF
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
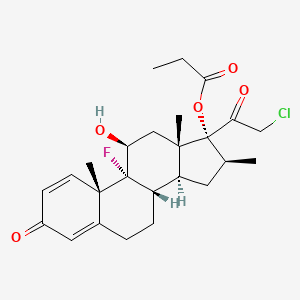

1. Clobetasol
2. Clobetasol 17 Propionate
3. Clobetasol 17-propionate
4. Clobex
5. Clofenazon
6. Cormax
7. Dermovate
8. Embeline
9. Embeline E
10. Olux
11. Temovate
1. 25122-46-7
2. Clobetasol 17-propionate
3. Clobex
4. Dermovate
5. Temovate
6. Embeline
7. Cormax
8. Olux
9. Gr 2/925
10. Cci 4725
11. Clobestasol Propionate
12. Impoyz
13. Psorex
14. [(8s,9r,10s,11s,13s,14s,16s,17r)-17-(2-chloroacetyl)-9-fluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] Propanoate
15. Clobetasol 17-propanoate
16. Clobetasol-17-propionate
17. Mls000028708
18. Cci-4725
19. 21-chloro-9-fluoro-11beta,17-dihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17-propionate
20. Embeline E
21. Smr000058745
22. Pregna-1,4-diene-3,20-dione, 21-chloro-9-fluoro-11-hydroxy-16-methyl-17-(1-oxopropoxy)-, (11beta,16beta)-
23. Clobesol
24. Chebi:31414
25. Temovate E
26. Nsc-758634
27. Dsstox_cid_25907
28. Dsstox_rid_81219
29. Dsstox_gsid_45907
30. Olux-e
31. 779619577m
32. Dermoxinale
33. Butavate
34. Dermoval
35. Yugofin
36. 21-chloro-9-fluoro-11beta-hydroxy-16beta-methyl-3,20-dioxopregna-1,4-dien-17-yl Propanoate
37. Olux E
38. Cgp 9555
39. Clobetasol Propionate Gel
40. Einecs 246-634-3
41. Clobetasol Propionate (emollient)
42. Clarelux
43. Ncgc00095078-01
44. Pregna-1,4-diene-3,20-dione, 21-chloro-9-fluoro-11-hydroxy-16-methyl-17-(1-oxopropoxy)-, (11.beta.,16.beta.)-
45. Clobetasol-propionate
46. Cas-25122-46-7
47. Clobex (tn)
48. Cormax (tn)
49. Mfcd00058499
50. Unii-779619577m
51. Impeklo
52. Clobetasol Propionate [usan:usp:jan]
53. Olux (tn)
54. Clobetasol Propionate E
55. Propionic Acid Clobetasol
56. Opera_id_1470
57. Schembl3997
58. 21-chloro-9-fluoro-11-hydroxy-16-methyl-17-(1-oxopropoxy)pregna-1,4-diene-3,20-dione (11beta,16beta)-
59. Cid_32798
60. Mls001076345
61. Amot0927
62. Gtpl7062
63. Ccl 4725
64. Chembl1159650
65. Dtxsid6045907
66. Bdbm39347
67. Cgp9555
68. Clobetasol Propionate [mi]
69. Hms2090a04
70. Hms2230h08
71. Hms3259a10
72. Hms3714m04
73. Clobetasol Propionate (jp17/usp)
74. Clobetasol Propionate [jan]
75. Bcp04125
76. Zinc3977767
77. Clobetasol Propionate [usan]
78. Tox21_111414
79. Am9637
80. Clobetasol Propionate [vandf]
81. S2584
82. Clobetasol Propionate [mart.]
83. Akos015951278
84. Clobetasol Propionate [usp-rs]
85. Clobetasol Propionate [who-dd]
86. Tox21_111414_1
87. Bcp9000539
88. Ccg-220781
89. Clobetasol 17-propionate [mi]
90. Db01013
91. Nc00551
92. Nsc 758634
93. 21-chloro-9-fluoro-11beta-hydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17-propionate
94. Ncgc00023373-03
95. Ncgc00023373-04
96. Ac-17990
97. As-12555
98. Clobetasol Propionate, Analytical Standard
99. Cpd000058745
100. Gr-2/925
101. Hy-13600
102. Pregna-1,4-diene-3,20-dione, 21-chloro-9-fluoro-11-beta,17-dihydroxy-16-beta-methyl-, 17-propionate
103. Bcp0726000094
104. Clobetasol Propionate [orange Book]
105. Clobetasol Propionate [usp Impurity]
106. Clobetasol Propionate [usp Monograph]
107. D01272
108. D94623
109. Ab00383081-10
110. Ab00383081_11
111. 122c467
112. Clobetasol Propionate 1000 Microg/ml In Methanol
113. Q421637
114. Sr-01000000280
115. Clobetasol Propionate 100 Microg/ml In Acetonitrile
116. Sr-01000000280-4
117. Brd-k10799896-001-10-6
118. Brd-k10799896-001-26-2
119. Clobetasol Propionate 100 Microg/ml In Methanol/water
120. Clobetasol Propionate, European Pharmacopoeia (ep) Reference Standard
121. Clobetasol Propionate, United States Pharmacopeia (usp) Reference Standard
122. Clobetasol For Peak Identification, European Pharmacopoeia (ep) Reference Standard
123. Clobetasol Propionate, Pharmaceutical Secondary Standard; Certified Reference Material
124. (8alpha,11beta,14beta,16alpha,17alpha)-21-chloro-9-fluoro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-yl Propanoate
125. 21-chloro-9-fluoro-11.beta.,17-dihydroxy-16.beta.-methylpregna-1,4-diene-3,20-dione 17-propionate
126. 21-chloro-9alpha-fluoro-11beta,17alpha-dihydroxy-16beta-methyl-1,4-pregnadiene-3,20-dione 17-propionate
127. Cgp9555 Pound>> Ccl 4725 Pound>>cgp 9555 Pound>> Ccl4725 Pound>>cgp-9555 Pound>> Ccl-4725
128. Xrd
| Molecular Weight | 467.0 g/mol |
|---|---|
| Molecular Formula | C25H32ClFO5 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 466.1922300 g/mol |
| Monoisotopic Mass | 466.1922300 g/mol |
| Topological Polar Surface Area | 80.7 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 929 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 20 | |
|---|---|
| Drug Name | Clobetasol propionate |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Lotion; Gel; Shampoo; Ointment; Cream; Spray; Aerosol, foam; Solution |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Wockhardt; Fougera Pharms; Teva Pharms; Paddock; Actavis Mid Atlantic; Perrigo; Taro; Fougera; Tolmar; Perrigo Israel |
| 2 of 20 | |
|---|---|
| Drug Name | Clobetasol propionate |
| PubMed Health | Clobetasol Propionate (On the skin) |
| Drug Classes | Corticosteroid, Very Strong |
| Drug Label | Clobetasol propionate cream USP, 0.05% (emollient) contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degre... |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Fougera Pharms; Taro |
| 3 of 20 | |
|---|---|
| Drug Name | Clobex |
| Drug Label | CLOBEX (clobetasol propionate) Lotion, 0.05% contains clobetasol propionate, a synthetic fluorinated corticosteroid, for topical dermatologic use. The corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflamm... |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Lotion; Shampoo; Spray |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Galderma Labs |
| 4 of 20 | |
|---|---|
| Drug Name | Cormax |
| PubMed Health | Clobetasol Propionate (On the skin) |
| Drug Classes | Corticosteroid, Very Strong |
| Drug Label | Cormax Ointment contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical dermatologic use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid... |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Hi Tech Pharma |
| 5 of 20 | |
|---|---|
| Drug Name | Embeline |
| Drug Label | Clobetasol propionate foam, 0.05% contains clobetasol propionate, USP, a synthetic corticosteroid, for topical dermatologic use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocortic... |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Gel; Ointment; Solution |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Hi Tech Pharma |
| 6 of 20 | |
|---|---|
| Drug Name | Embeline e |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Hi Tech Pharma |
| 7 of 20 | |
|---|---|
| Drug Name | Olux |
| PubMed Health | Clobetasol |
| Drug Classes | Corticosteroid, Very Strong, Corticosteroid, Weak |
| Drug Label | OLUX-E (clobetasol propionate) Foam, 0.05% is a white to off-white petrolatum-based emulsion aerosol foam containing the active ingredient clobetasol propionate USP, a synthetic corticosteroid for topical dermatologic use. Clobetasol, an analog of pr... |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Aerosol, foam |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Delcor Asset |
| 8 of 20 | |
|---|---|
| Drug Name | Olux e |
| PubMed Health | Clobetasol |
| Drug Classes | Corticosteroid, Very Strong, Corticosteroid, Weak |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Aerosol, foam |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Delcor Asset |
| 9 of 20 | |
|---|---|
| Drug Name | Temovate |
| PubMed Health | Clobetasol |
| Drug Classes | Corticosteroid, Very Strong, Corticosteroid, Weak |
| Drug Label | Temovate E (clobetasol propionate) Cream, 0.05% contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree... |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Ointment; Cream; Gel |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Fougera Pharms |
| 10 of 20 | |
|---|---|
| Drug Name | Temovate e |
| PubMed Health | Clobetasol Propionate (On the skin) |
| Drug Classes | Corticosteroid, Very Strong |
| Drug Label | Temovate E (clobetasol propionate) Cream, 0.05% contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree... |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Fougera Pharms |
| 11 of 20 | |
|---|---|
| Drug Name | Clobetasol propionate |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Lotion; Gel; Shampoo; Ointment; Cream; Spray; Aerosol, foam; Solution |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Wockhardt; Fougera Pharms; Teva Pharms; Paddock; Actavis Mid Atlantic; Perrigo; Taro; Fougera; Tolmar; Perrigo Israel |
| 12 of 20 | |
|---|---|
| Drug Name | Clobetasol propionate |
| PubMed Health | Clobetasol Propionate (On the skin) |
| Drug Classes | Corticosteroid, Very Strong |
| Drug Label | Clobetasol propionate cream USP, 0.05% (emollient) contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degre... |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Fougera Pharms; Taro |
| 13 of 20 | |
|---|---|
| Drug Name | Clobex |
| Drug Label | CLOBEX (clobetasol propionate) Lotion, 0.05% contains clobetasol propionate, a synthetic fluorinated corticosteroid, for topical dermatologic use. The corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflamm... |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Lotion; Shampoo; Spray |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Galderma Labs |
| 14 of 20 | |
|---|---|
| Drug Name | Cormax |
| PubMed Health | Clobetasol Propionate (On the skin) |
| Drug Classes | Corticosteroid, Very Strong |
| Drug Label | Cormax Ointment contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical dermatologic use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocorticoid... |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Hi Tech Pharma |
| 15 of 20 | |
|---|---|
| Drug Name | Embeline |
| Drug Label | Clobetasol propionate foam, 0.05% contains clobetasol propionate, USP, a synthetic corticosteroid, for topical dermatologic use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree of mineralocortic... |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Gel; Ointment; Solution |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Hi Tech Pharma |
| 16 of 20 | |
|---|---|
| Drug Name | Embeline e |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Hi Tech Pharma |
| 17 of 20 | |
|---|---|
| Drug Name | Olux |
| PubMed Health | Clobetasol |
| Drug Classes | Corticosteroid, Very Strong, Corticosteroid, Weak |
| Drug Label | OLUX-E (clobetasol propionate) Foam, 0.05% is a white to off-white petrolatum-based emulsion aerosol foam containing the active ingredient clobetasol propionate USP, a synthetic corticosteroid for topical dermatologic use. Clobetasol, an analog of pr... |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Aerosol, foam |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Delcor Asset |
| 18 of 20 | |
|---|---|
| Drug Name | Olux e |
| PubMed Health | Clobetasol |
| Drug Classes | Corticosteroid, Very Strong, Corticosteroid, Weak |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Aerosol, foam |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Delcor Asset |
| 19 of 20 | |
|---|---|
| Drug Name | Temovate |
| PubMed Health | Clobetasol |
| Drug Classes | Corticosteroid, Very Strong, Corticosteroid, Weak |
| Drug Label | Temovate E (clobetasol propionate) Cream, 0.05% contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree... |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Ointment; Cream; Gel |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Fougera Pharms |
| 20 of 20 | |
|---|---|
| Drug Name | Temovate e |
| PubMed Health | Clobetasol Propionate (On the skin) |
| Drug Classes | Corticosteroid, Very Strong |
| Drug Label | Temovate E (clobetasol propionate) Cream, 0.05% contains the active compound clobetasol propionate, a synthetic corticosteroid, for topical use. Clobetasol, an analog of prednisolone, has a high degree of glucocorticoid activity and a slight degree... |
| Active Ingredient | Clobetasol propionate |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Fougera Pharms |
Clobetasol propionate is indicated to treat moderate to severe plaque psoriasis as well as inflammatory and pruritic manifestations of corticosteroid responsive dermatoses.
FDA Label
Corticosteroids bind to the glucocorticoid receptor, inhibiting pro-inflammatory signals, and promoting anti-inflammatory signals. Clobetasol propionate is generally applied twice daily so the duration of action is long. Corticosteroids have a wide therapeutic window as patients may require doses that are multiples of what the body naturally produces. Patients taking corticosteroids should be counselled regarding the risk of hypothalamic-pituitary-adrenal axis suppression and increased susceptibility to infections.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
Absorption
Twice daily application of clobetasol foam leads to a Cmax of 5936pg/mL with a Tmax of 5 hours. Clobetasol cream showed an increase in clobetasol concentrations from 50.796.0pg/mL to 56.3104.7pg/mL.
Route of Elimination
Corticosteroids are eliminated predominantly in the urine.
Volume of Distribution
Data regarding the volume of distribution of clobetasole propionate are not readily available.
Clearance
Data regarding the clearance of clobetasol propionate are not readily available.
The metabolism of clobetasol propionate is not well studied but it does induce metabolic enzymes, even when delivered topically. The metabolism of clobetasol propionate is predicted to follow similar metabolic pathways to other corticosteroids including the addition of oxygen, hydrogen, glucuronides, and sulfates to form water soluble metabolites.
Data regarding the half life of clobetasol propionate are not readily available.
The short term effects of corticosteroids are decreased vasodilation and permeability of capillaries, as well as decreased leukocyte migration to sites of inflammation. Corticosteroids binding to the glucocorticoid receptor mediates changes in gene expression that lead to multiple downstream effects over hours to days. Glucocorticoids inhibit neutrophil apoptosis and demargination; they inhibit phospholipase A2, which decreases the formation of arachidonic acid derivatives; they inhibit NF-Kappa B and other inflammatory transcription factors; they promote anti-inflammatory genes like interleukin-10. Lower doses of corticosteroids provide an anti-inflammatory effect, while higher doses are immunosuppressive. High doses of glucocorticoids for an extended period bind to the mineralocorticoid receptor, raising sodium levels and decreasing potassium levels.
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2013-04-11
Pay. Date : 2012-12-31
DMF Number : 20821
Submission : 2007-08-29
Status : Active
Type : II

Certificate Number : R1-CEP 2007-263 - Rev 02
Issue Date : 2016-01-22
Type : Chemical
Substance Number : 2127
Status : Valid

Registration Number : 229MF10086
Registrant's Address : 385/2, Pigdamber, Off. A. B. Road, Rau, Indore 453331 (M.P.) India
Initial Date of Registration : 2017-04-25
Latest Date of Registration :

Date of Issue : 2025-09-03
Valid Till : 2028-09-02
Written Confirmation Number : WC-0161
Address of the Firm :

Registrant Name : GlaxoSmithKline Inc.
Registration Date : 2021-11-10
Registration Number : 20121126-93-E-103-07(A)
Manufacturer Name : Symbiotec Pharmalab Pvt. Limited
Manufacturer Address : 385/2 Gram Pigdamber, Near Hotel Mashal, Off AB Road, Rau, Indore-453 331 (MP) India

 Cerata Pharmaceuticals LLP: WHO-GMP Certified Leading Manufacturer & Exporter of Steroid-Hormone & Peptide APIs From India.
Cerata Pharmaceuticals LLP: WHO-GMP Certified Leading Manufacturer & Exporter of Steroid-Hormone & Peptide APIs From India.

GDUFA
DMF Review : Reviewed
Rev. Date : 2015-02-23
Pay. Date : 2015-02-17
DMF Number : 8336
Submission : 1989-12-09
Status : Active
Type : II

Registration Number : 222MF10130
Registrant's Address : Via Pavia, 1-27027 Gropello Cairoli, Pavia, Italy
Initial Date of Registration : 2010-04-12
Latest Date of Registration :

NDC Package Code : 46439-8784
Start Marketing Date : 2025-02-20
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT

Registrant Name : Galderma Korea Co., Ltd.
Registration Date : 2012-11-22
Registration Number : 20121123-93-E-100-06
Manufacturer Name : Farmabios SpA
Manufacturer Address : via Pavia 1 27027 GROPELLO CAIROLI (PV) (microchem SRL, Via Turati 2 IT-29017 Fiorenzuola D'Arda (PC), Italy)

| Available Reg Filing : SAU |
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.
IKF/Pharmasynthese have been with fine chemicals market and APIs performance for more than 40 years.

Date of Issue : 2025-11-20
Valid Till : 2028-11-19
Written Confirmation Number : WC-0009
Address of the Firm :
 Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
Gonane has API manufacturing expertise in new-age Corticosteroids, Hormones and other pharma raw materials.
 Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
Vamsi Labs is one of the major manufacturers of Anti-asthmatic, Anti-migraine & Anti-psychotic APIs.
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
78
PharmaCompass offers a list of Clobetasol Propionate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Clobetasol Propionate manufacturer or Clobetasol Propionate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Clobetasol Propionate manufacturer or Clobetasol Propionate supplier.
PharmaCompass also assists you with knowing the Clobetasol Propionate API Price utilized in the formulation of products. Clobetasol Propionate API Price is not always fixed or binding as the Clobetasol Propionate Price is obtained through a variety of data sources. The Clobetasol Propionate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Clobetasol Propionate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Clobetasol Propionate, including repackagers and relabelers. The FDA regulates Clobetasol Propionate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Clobetasol Propionate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Clobetasol Propionate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Clobetasol Propionate supplier is an individual or a company that provides Clobetasol Propionate active pharmaceutical ingredient (API) or Clobetasol Propionate finished formulations upon request. The Clobetasol Propionate suppliers may include Clobetasol Propionate API manufacturers, exporters, distributors and traders.
click here to find a list of Clobetasol Propionate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Clobetasol Propionate DMF (Drug Master File) is a document detailing the whole manufacturing process of Clobetasol Propionate active pharmaceutical ingredient (API) in detail. Different forms of Clobetasol Propionate DMFs exist exist since differing nations have different regulations, such as Clobetasol Propionate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Clobetasol Propionate DMF submitted to regulatory agencies in the US is known as a USDMF. Clobetasol Propionate USDMF includes data on Clobetasol Propionate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Clobetasol Propionate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Clobetasol Propionate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Clobetasol Propionate Drug Master File in Japan (Clobetasol Propionate JDMF) empowers Clobetasol Propionate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Clobetasol Propionate JDMF during the approval evaluation for pharmaceutical products. At the time of Clobetasol Propionate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Clobetasol Propionate suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Clobetasol Propionate Drug Master File in Korea (Clobetasol Propionate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Clobetasol Propionate. The MFDS reviews the Clobetasol Propionate KDMF as part of the drug registration process and uses the information provided in the Clobetasol Propionate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Clobetasol Propionate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Clobetasol Propionate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Clobetasol Propionate suppliers with KDMF on PharmaCompass.
A Clobetasol Propionate CEP of the European Pharmacopoeia monograph is often referred to as a Clobetasol Propionate Certificate of Suitability (COS). The purpose of a Clobetasol Propionate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Clobetasol Propionate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Clobetasol Propionate to their clients by showing that a Clobetasol Propionate CEP has been issued for it. The manufacturer submits a Clobetasol Propionate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Clobetasol Propionate CEP holder for the record. Additionally, the data presented in the Clobetasol Propionate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Clobetasol Propionate DMF.
A Clobetasol Propionate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Clobetasol Propionate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Clobetasol Propionate suppliers with CEP (COS) on PharmaCompass.
A Clobetasol Propionate written confirmation (Clobetasol Propionate WC) is an official document issued by a regulatory agency to a Clobetasol Propionate manufacturer, verifying that the manufacturing facility of a Clobetasol Propionate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Clobetasol Propionate APIs or Clobetasol Propionate finished pharmaceutical products to another nation, regulatory agencies frequently require a Clobetasol Propionate WC (written confirmation) as part of the regulatory process.
click here to find a list of Clobetasol Propionate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Clobetasol Propionate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Clobetasol Propionate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Clobetasol Propionate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Clobetasol Propionate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Clobetasol Propionate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Clobetasol Propionate suppliers with NDC on PharmaCompass.
Clobetasol Propionate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Clobetasol Propionate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Clobetasol Propionate GMP manufacturer or Clobetasol Propionate GMP API supplier for your needs.
A Clobetasol Propionate CoA (Certificate of Analysis) is a formal document that attests to Clobetasol Propionate's compliance with Clobetasol Propionate specifications and serves as a tool for batch-level quality control.
Clobetasol Propionate CoA mostly includes findings from lab analyses of a specific batch. For each Clobetasol Propionate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Clobetasol Propionate may be tested according to a variety of international standards, such as European Pharmacopoeia (Clobetasol Propionate EP), Clobetasol Propionate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Clobetasol Propionate USP).

