
Synopsis
Synopsis
0
CEP/COS
0
VMF
0
Australia
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
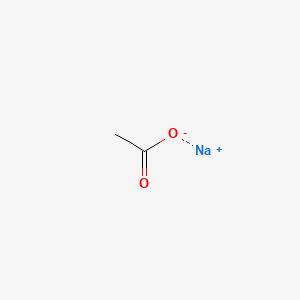

1. Sodium Acetate Trihydrate
2. Sodium Acetate, Anhydrous
1. 127-09-3
2. Acetic Acid, Sodium Salt
3. Sodium Acetate Anhydrous
4. Acetic Acid Sodium Salt
5. Sodium Acetate, Anhydrous
6. Anhydrous Sodium Acetate
7. Sodium Ethanoate
8. Fema No. 3024
9. Sodium-acetate
10. Acetic Acid, Sodium Salt (1:1)
11. Sodium;acetate
12. Mfcd00012459
13. Natriumacetat
14. Sodium Acetate,anhydrous
15. Nvg71zz7p0
16. Chebi:32954
17. Sodii Acetas
18. Natriumazetat
19. Nsc-77459
20. Natrium Aceticum
21. Octan Sodny [czech]
22. Caswell No. 741a
23. Natriumacetat [german]
24. Chembl1354
25. Fema Number 3024
26. Octan Sodny
27. Naoac
28. Hsdb 688
29. Sodium Acetate In Plastic Container
30. Einecs 204-823-8
31. Nsc 77459
32. Unii-nvg71zz7p0
33. Epa Pesticide Chemical Code 044006
34. Sodiumacetate
35. Sodium Aceate
36. Acona
37. Scfa
38. Ch3coona
39. Acetic Acidsodium Salt
40. Sodium Acetate Solution
41. Short Chain Fatty Acids
42. Sodium Acetate ,(s)
43. Ch3co2na
44. Dsstox_cid_7044
45. Ec 204-823-8
46. Dsstox_rid_78290
47. Sodium Acetate [mi]
48. Dsstox_gsid_27044
49. Sodium Acetate, Acs Reagent
50. Sodium Acetate [fhfi]
51. Sodium Acetate [hsdb]
52. Dtxsid2027044
53. Sodium Acetate [who-dd]
54. Sodium Acetate Solution, 0.3 M
55. Sodium Acetate, Biochemical Grade
56. Sodium Acetate Anhydrous Acs Usp
57. Tox21_202741
58. Sodium Acetate Anhydrous [ii]
59. Akos003052995
60. Akos015837569
61. Sodium Acetate, Bioxtra, >=99.0%
62. Db09395
63. Sodium Acetate, Reagentplus(r), 99%
64. Sodium Acetate, For Hplc, >=99.5%
65. Sodium Acetate,anhydrous [vandf]
66. Acetate, 1m Buffer Solution, Ph, 3.0
67. Acetate, 1m Buffer Solution, Ph, 3.5
68. Acetate, 1m Buffer Solution, Ph, 4.0
69. Acetate, 1m Buffer Solution, Ph, 5.0
70. Acetate, 1m Buffer Solution, Ph, 5.5
71. Ncgc00260289-01
72. Sodium Acetate, Ar, Anhydrous, >=99%
73. Sodium Acetate, Lr, Anhydrous, >=98%
74. Cas-127-09-3
75. E262
76. Sodium Acetate, Acs Reagent, >=99.0%
77. B7296
78. Ft-0635282
79. Ft-0659959
80. Ft-0689166
81. S0559
82. Sodium Acetate Anhydrous, >99%, Fcc, Fg
83. Sodium Acetate Anhydrous [orange Book]
84. Sodium Acetate, 99.995% Trace Metals Basis
85. Sodium Acetate, Saj First Grade, >=98.0%
86. Sodium Acetate, Trace Metals Grade, 99.99%
87. Sodium Acetate Anhydrous Acs Grade 12kg
88. Sodium Acetate, Jis Special Grade, >=98.5%
89. Sodium Acetate, Vetec(tm) Reagent Grade, 98%
90. Sodium Acetate Anhydrous [usp Monograph]
91. A805637
92. Q339940
93. J-005463
94. Sodium Acetate, For Hplc, 99.0-101.0% (nt)
95. Sodium Acetate, Puriss., Anhydrous, >=98%, Powder
96. Sodium Acetate, Anhydrous, Reagentplus(r), >=99.0%
97. Sodium Acetate, Anhydrous, For Molecular Biology, >=99%
98. Sodium Acetate, For Electrophoresis, >=99%, Crystalline
99. Sodium Acetate, 1m Aqueous Solution, Ph 4.5, Rnase Free
100. Sodium Acetate, 3m Aqueous Solution, Ph 4.5, Autoclaved
101. Sodium Acetate, 3m Aqueous Solution, Ph 5.2, Autoclaved
102. Sodium Acetate, 3m Aqueous Solution, Ph 5.2, Rnase Free
103. Sodium Acetate, 3m Aqueous Solution, Ph 7.0, Autoclaved
104. Sodium Acetate, 3m Aqueous Solution, Ph 7.0, Rnase Free
105. Sodium Acetate, Meets Usp Testing Specifications, Anhydrous
106. Sodium Acetate, United States Pharmacopeia (usp) Reference Standard
107. Sodium Acetate Solution, Bioultra, For Molecular Biology, ~3 M In H2o
108. Sodium Acetate, Bioultra, For Luminescence, Anhydrous, >=99.0% (nt)
109. Sodium Acetate, Puriss. P.a., Acs Reagent, Reag. Ph. Eur., Anhydrous
110. Sodium Acetate, Anhydrous, Bioultra, For Luminescence, For Molecular Biology, >=99.0% (nt)
111. Sodium Acetate, Anhydrous, Free-flowing, Redi-dri(tm), Acs Reagent, >=99.0%
112. Mettler-toledo Calibration Substance Me 30130599, Sodium Acetate Anhydrous, Tracable To Primary Standards (lgc)
113. Sodium Acetate Solution, Nmr Reference Standard, 50 Mm In D2o (99.9 Atom % D), Water 1 %, Nmr Tube Size 3 Mm X 8 In.
114. Sodium Acetate, Powder, Bioreagent, For Electrophoresis, Suitable For Cell Culture, Suitable For Insect Cell Culture, >=99%
| Molecular Weight | 82.03 g/mol |
|---|---|
| Molecular Formula | C2H3NaO2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 82.00307362 g/mol |
| Monoisotopic Mass | 82.00307362 g/mol |
| Topological Polar Surface Area | 40.1 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 34.6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Sodium acetate in plastic container |
| Active Ingredient | Sodium acetate anhydrous |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2meq/ml |
| Market Status | Prescription |
| Company | Hospira |
| 2 of 2 | |
|---|---|
| Drug Name | Sodium acetate in plastic container |
| Active Ingredient | Sodium acetate anhydrous |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2meq/ml |
| Market Status | Prescription |
| Company | Hospira |
Diuretics; Expectorants; Pharmaceutic Aids
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/FORMER USE/: HYOSCYAMUS SODIUM ACETATE, & PHENOBARBITAL ELIXIR COMBINES SMOOTH MUSCLE ANTISPASMODIC ACTION OF ... HYOSCYAMUS, WITH URINARY ALKALINIZING & DIURETIC EFFECTS OF SODIUM ACETATE, & SEDATIVE EFFECTS OF PHENOBARBITAL. /IT IS/ USED IN MANAGEMENT OF CYSTITIS & FOR BLADDER IRRITATION.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 86:00
DOSAGE OF ELIXIR OF HYOSCYAMUS CMPD /CONTAINING SODIUM ACETATE & PHENOBARBITAL/ IS 5 ML GIVEN 3 TIMES DAILY BEFORE MEALS.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 86:00
... /SODIUM ACETATE/ HAS BEEN USED FOR PARENTERAL THERAPY OF ACIDOTIC CONDITIONS. IT IS BOTH A SYSTEMIC & URINARY ALKALIZER. /SODIUM ACETATE TRIHYDRATE, USP/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 772
For more Therapeutic Uses (Complete) data for SODIUM ACETATE (8 total), please visit the HSDB record page.
Injection, USP 40 mEq is indicated as a source of sodium, for addition to large volume intravenous fluids to prevent or correct hyponatremia in patients with restricted or no oral intake. It is also useful as an additive for preparing specific intravenous fluid formulas when the needs of the patient cannot be met by standard electrolyte or nutrient solutions. Sodium acetate and other bicarbonate precursors are alkalinising agents, and can be used to correct metabolic acidosis, or for alkalinisation of the urine.
Sodium is the principal cation of extracellular fluid. It comprises more than 90% of total cations at its normal plasma concentration of approximately 140 mEq/liter. The sodium ion exerts a primary role in controlling total body water and its distribution. Acetate ions acts as hydrogen ion acceptor which is alternative to bicarbonate.
B - Blood and blood forming organs
B05 - Blood substitutes and perfusion solutions
B05X - I.v. solution additives
B05XA - Electrolyte solutions
B05XA08 - Sodium acetate
Absorption
It is readily available in the circulation after IV administration.
Route of Elimination
Both the sodium and bicarbonate ions are excreted mainly in the urine. Some sodium is excreted in the feces, and small amounts may also be excreted in saliva, sweat, bile and pancreatic secretions.
In liver, sodium acetate is being metabolized into bicarbonate. To form bicarbonate, acetate is slowly hydrolyzed to carbon dioxide and water, which are then converted to bicarbonate by the addition of a hydrogen ion.
THE ACETATE ION IS RAPIDLY & COMPLETELY METABOLIZED BY BODY, & CONSEQUENTLY ADMIN OF SODIUM ACETATE IS EVENTUALLY EQUIVALENT TO GIVING SODIUM BICARBONATE. /SODIUM ACETATE TRIHYDRATE, USP/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 772
CONSTANT RATE INFUSIONS OF SODIUM ACETATE GIVEN TO VOLUNTEERS, AT 4 MMOL/KG/HR INCR PLASMA ACETATE BY ONLY 0.41 MMOL/L, INDICATING A HIGH ACETATE METABOLIZING CAPACITY IN MAN.
KVEIM MH R ET AL; J OSLO CITY HOSP: 30 (8): 101 (1980)
Readily metabolized outside the liver.
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 723
It works as a source of sodium ions especially in cases of hyponatremic patients. Sodium has a primary role in regulating extracellular fluid volume. It controls water distribution, fluid and electrolyte balance and the osmotic pressure of body fluids. Sodium is also involved in nerve conduction, muscle contraction, acid-base balance and cell nutrient uptake.
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
59
PharmaCompass offers a list of Sodium Acetate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Sodium Acetate manufacturer or Sodium Acetate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Sodium Acetate manufacturer or Sodium Acetate supplier.
PharmaCompass also assists you with knowing the Sodium Acetate API Price utilized in the formulation of products. Sodium Acetate API Price is not always fixed or binding as the Sodium Acetate Price is obtained through a variety of data sources. The Sodium Acetate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5, including repackagers and relabelers. The FDA regulates CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 supplier is an individual or a company that provides CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 active pharmaceutical ingredient (API) or CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 finished formulations upon request. The CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 suppliers may include CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 API manufacturers, exporters, distributors and traders.
click here to find a list of CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 DMF (Drug Master File) is a document detailing the whole manufacturing process of CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 active pharmaceutical ingredient (API) in detail. Different forms of CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 DMFs exist exist since differing nations have different regulations, such as CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 DMF submitted to regulatory agencies in the US is known as a USDMF. CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 USDMF includes data on CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 Drug Master File in Japan (CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 JDMF) empowers CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 JDMF during the approval evaluation for pharmaceutical products. At the time of CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 Drug Master File in Korea (CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5. The MFDS reviews the CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 KDMF as part of the drug registration process and uses the information provided in the CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 KDMF to evaluate the safety and efficacy of the drug.
After submitting a CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 API can apply through the Korea Drug Master File (KDMF).
click here to find a list of CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 suppliers with KDMF on PharmaCompass.
A CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 written confirmation (CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 WC) is an official document issued by a regulatory agency to a CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 manufacturer, verifying that the manufacturing facility of a CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 APIs or CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 finished pharmaceutical products to another nation, regulatory agencies frequently require a CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 WC (written confirmation) as part of the regulatory process.
click here to find a list of CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 suppliers with NDC on PharmaCompass.
CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 GMP manufacturer or CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 GMP API supplier for your needs.
A CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 CoA (Certificate of Analysis) is a formal document that attests to CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5's compliance with CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 specifications and serves as a tool for batch-level quality control.
CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 CoA mostly includes findings from lab analyses of a specific batch. For each CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 may be tested according to a variety of international standards, such as European Pharmacopoeia (CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 EP), CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (CLINIMIX E 5/20 SULFITE-FREE W/ ELECT IN 20% DEXTROSE W/ CALCIUM IN PLASTIC CONTAINER-5 USP).

