



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
0
Other Suppliers

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
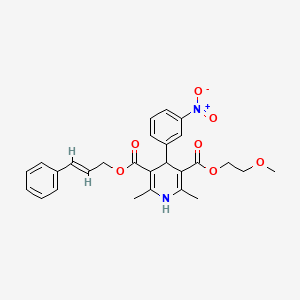

1. 2-methoxyethyl-3-phenyl-2-propen-1-yl-1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)pyridine-3,5-dicarboxylate
2. Cilnidipine, (+)-isomer
3. Cilnidipine, (-)-isomer
4. Frc 8653
5. Frc-8653
1. 132203-70-4
2. Cinalong
3. Atelec
4. Siscard
5. Frc-8653
6. Frc 8653
7. 3-cinnamyl 5-(2-methoxyethyl) 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
8. Cilnidipine [inn]
9. Chebi:31399
10. Cilnidipine, (+)-
11. Cilnidipine, (-)-
12. (+-)-(e)-cinnamyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate
13. 97t5az1jip
14. 4lnu2su262
15. S85436zg85
16. 2-methoxyethyl (2e)-3-phenylprop-2-en-1-yl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
17. Ncgc00162150-01
18. Cinaldipine
19. (+)-frc-8653
20. (-)-frc-8653
21. Dsstox_cid_26309
22. Dsstox_rid_81530
23. Dsstox_gsid_46309
24. (e)-cinnamyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate
25. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 2-methoxyethyl 3-phenyl-2-propenyl Ester, (e)-(+)-
26. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 2-methoxyethyl 3-phenyl-2-propenyl Ester, (e)-(-)-
27. Atelec (tn)
28. Cas-132203-70-4
29. Sr-05000001454
30. 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic Acid 2-methoxyethyl (2e)-3-phenyl-2-propenyl Ester
31. Unii-97t5az1jip
32. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 3-(2-methoxyethyl) 5-((2e)-3-phenyl-2-propen-1-yl) Ester
33. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 3-(2-methoxyethyl) 5-[(2e)-3-phenyl-2-propen-1-yl] Ester
34. Cilnidipine- Bio-x
35. Mfcd00865853
36. Cilnidipine [mi]
37. Cilnidipine [jan]
38. Cilnidipine (jp17/inn)
39. Cilnidipine [mart.]
40. Unii-4lnu2su262
41. Schembl25550
42. Cilnidipine [who-dd]
43. 3-o-(2-methoxyethyl) 5-o-[(e)-3-phenylprop-2-enyl] 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
44. Chembl452076
45. Gtpl7767
46. Dtxsid0046309
47. Unii-s85436zg85
48. Chebi:91506
49. Hms2089j07
50. Hms3261e06
51. Hms3413l13
52. Hms3677l13
53. Hms3715n17
54. Hms3884k09
55. Bcp22689
56. Tox21_112001
57. Tox21_500422
58. Ac-270
59. Bdbm50101813
60. S1293
61. Stk623341
62. Akos005558085
63. Tox21_112001_1
64. Ccg-221188
65. Ccg-221726
66. Cilnidipine, >=98% (hplc), Powder
67. Cs-1133
68. Db09232
69. Ks-1294
70. Lp00422
71. Sdccgsbi-0633712.p001
72. Ncgc00162150-02
73. Ncgc00162150-03
74. Ncgc00162150-04
75. Ncgc00162150-16
76. Ncgc00261107-01
77. 3,5-pyridinedicarboxylic Acid, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 2-methoxyethyl 3-phenyl-2-propenyl Ester, (e)-(+-)-
78. Bc164309
79. Hy-17404
80. Ls-15175
81. Sw219784-1
82. D01173
83. T70209
84. Ab01274755-01
85. Ab01274755-02
86. Ab01274755_03
87. Frc-8653; Frc 8653; Frc8653
88. 203c704
89. Q731525
90. J-006141
91. Sr-05000001454-1
92. Sr-05000001454-2
93. Brd-a07875874-001-01-6
94. F2173-0669
95. (+/-)-(e)-cinnamyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate
96. 102106-21-8
97. 118934-76-2
98. 118934-77-3
99. 132295-21-7
100. 132338-87-5
101. 2-methoxyethyl (2e)-3-phenyl-2-propenyl 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate
102. O3-(2-methoxyethyl) O5-(3-phenylprop-2-enyl) 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
| Molecular Weight | 492.5 g/mol |
|---|---|
| Molecular Formula | C27H28N2O7 |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 11 |
| Exact Mass | 492.18965124 g/mol |
| Monoisotopic Mass | 492.18965124 g/mol |
| Topological Polar Surface Area | 120 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 896 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cilnidipine is indicated for the management of hypertension for end-organ protection. It is reported to be useful in elderly patients and in those with diabetes and albuminuria. Cilnidipine has been increasingly used in patients with chronic kidney disease Hypertension is the term used to describe the presence of high blood pressure. The blood pressure is generated by the force of the blood pumped from the heart against the blood vessels. Thus hypertension is caused when there is too much pressure on the blood vessels and this effect can damage the blood vessel.
Administration of cilnidipine has been shown to present an antisympathetic profile in vitro and in vivo. It decreases blood pressure safely and effectively without excessive blood pressure reduction or tachycardia.
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
C - Cardiovascular system
C08 - Calcium channel blockers
C08C - Selective calcium channel blockers with mainly vascular effects
C08CA - Dihydropyridine derivatives
C08CA14 - Cilnidipine
Absorption
Cilnidipine presents a very rapid absorption with a maximum peaked concentration after 2 hours. Its distribution tends to be higher in the liver as well as in kidneys, plasma and other tissues. Cilnidipine does not present a high accumulation in the tissue after repeated oral administration. Cilnidipine is reported to present very low bioavailability determined to be approximately 13%. This low bioavailability is suggested to be due to its low aqueous solubility and high permeability. Hence, efforts have been made in order to find an innovative formulation that can significantly improve the bioavailability of this drug. One of these formulations corresponds to the generation of polymeric nanoparticles which enhance the bioavailability by 2.5-3-fold.
Route of Elimination
Cilnidipine gets eliminated through the urine in a proportion of 20% of the administered dose and 80% is eliminated by the feces.
Volume of Distribution
Drugs on the group of dihydropyridines such as cilnidipine tend to have a large volume of distribution.
Cilnidipine is metabolized by both liver and kidney. It is rapidly metabolized by liver microsomes by a dehydrogenation process. The major enzymatic isoform involved in cilnidipine dehydrogenation of the dihydropyridine ring is CYP3A.
The half-life of the hypotensive effect for cilnidipine is of about 20.4 min.
Cilnidipine acts on the L-type calcium channels of blood vessels by blocking the incoming calcium and suppressing the contraction of blood vessels, thereby reducing blood pressure. Cilnidipine also works on the N-type calcium channel located at the end of the sympathetic nerve, inhibiting the emission of norepinephrine and suppressing the increase in stress blood pressure.


