



API Suppliers

US DMFs Filed
0

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
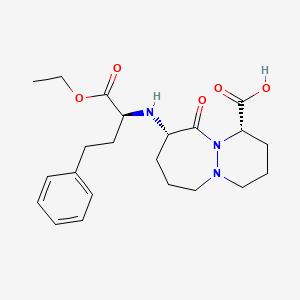

1. Cilazapril Anhydrous
2. Cilazapril Hydrate
3. Cilazapril Monohydrate
4. Cilazapril Monohydrobromide
5. Cilazapril, (s*)-isomer
6. Cilazapril, Anhydrous
7. Inhibace
8. Ro 31 2848
9. Ro 31-2848
10. Ro 312848
11. Ro-31-2848
12. Ro312848
1. 88768-40-5
2. Cilazapril Anhydrous
3. Inhibace
4. Cilazaprilum
5. Vascace
6. Cilazapril (anhydrous)
7. Dynorm
8. Cilazapril Hydrate
9. Cilazapril [inn]
10. Ro 34-2848
11. Anhydrous Cilazapril
12. Cilazapril (inn)
13. Inhibace (tn)
14. 6h-pyridazino(1,2-a)(1,2)diazepine-1-carboxylic Acid, 9-(((1s)-1-(ethoxycarbonyl)-3-phenylpropyl)amino)octahydro-10-oxo-, (1s,9s)-
15. Initiss
16. (4s,7s)-7-[[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]-6-oxo-1,2,3,4,7,8,9,10-octahydropyridazino[1,2-a]diazepine-4-carboxylic Acid
17. Ncgc00182039-01
18. Cilazaprilum [latin]
19. Ro-312848006
20. 8q9454114q
21. Cilazil
22. Inibace
23. Vascase
24. Unii-8q9454114q
25. Ro-312848
26. Cilazapril [mi]
27. Cilazapril [vandf]
28. Cilazapril [who-dd]
29. Dsstox_cid_28555
30. Dsstox_rid_82827
31. Dsstox_gsid_48629
32. Schembl24962
33. Chebi:3698
34. Chembl515606
35. Gtpl6459
36. Dtxsid1048629
37. Hy-a0043
38. Zinc3781951
39. Tox21_112971
40. Ac-269
41. Akos015951168
42. Db01340
43. Ro34-2848
44. Ncgc00182039-02
45. Ro-34-2848
46. (1s,9s)-9-{[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}-10-oxooctahydro-6h-pyridazino[1,2-a][1,2]diazepine-1-carboxylic Acid
47. 6h-pyridazino(1,2-a)(1,2)diazepine-1-carboxylic Acid, 9-((1-(ethoxycarbonyl)-3-phenylpropyl)amino)octahydro-10-oxo-, (1s-(1alpha,9alpha(r*)))-
48. 6h-pyridazino[1,2-a][1,2]diazepine-1-carboxylicacid, 9-[[(1s)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]octahydro-10-oxo-,(1s,9s)-
49. Cas-88768-40-5
50. Ro 31-2848006
51. D07699
52. Ab01565812_02
53. 768c405
54. A916338
55. Q867350
56. Ro-31-2848/006
57. Brd-k96177243-002-01-9
58. (1s,9s)-9-(((s)-1-carboxy-3-phenylpropyl)amino)octahydro-10-oxo-6h-pyridazino(1,2-a)(1,2)diazepine-1-carboxylic Acid 9-ethyl Ester
59. (1s,9s)-9-(((s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl)amino)-10-oxooctahydro-6h-pyridazino[1,2-a][1,2]diazepine-1-carboxylic Acid
60. (1s,9s)-9-[[(1s)-1-(ethoxycarbonyl)-3-phenylpropyl]amino]octahydro-10-oxo-6h-pyridazino[1,2-a][1,2]diazepine-1-carboxylic Acid
61. (1s,9s)-9-[[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]-10-oxo-1,2,3,4,6,7,8,9-octahydropyridazino[1,2-a]diazepine-1-carboxylic Acid
62. (1s,9s)-9-{[(2s)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}-10-oxo-octahydro-1h-pyridazino[1,2-a][1,2]diazepine-1-carboxylic Acid
63. (5s,8s)-5-{[(1s)-1-ethoxycarbonyl-3-phenyl-propyl]-amino}-6-oxo-1,7-diazabicyclo[5.4.0]undecane-8-carboxylic Acid
64. 6h-pyridazino(1,2-a)(1,2)diazepine-1-carboxylic Acid, 9-((1-(ethoxycarbonyl)-3-phenylpropyl)amino)octahydro-10-oxo-, Monohydrate, (1s-(1.alpha.,9.alpha.(r*)))
| Molecular Weight | 417.5 g/mol |
|---|---|
| Molecular Formula | C22H31N3O5 |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 9 |
| Exact Mass | 417.22637110 g/mol |
| Monoisotopic Mass | 417.22637110 g/mol |
| Topological Polar Surface Area | 99.2 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 608 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cilazapril is an ACE inhibtor class drug used in the treatment of hypertension and heart failure.
FDA Label
Cilazapril inhibits the production angiotensin II. By doing so, it decreases sodium and water reabsorption (via aldosterone) and it decreases vasoconstriction. The combined effect of this is a decrease in vascular resistance, and therefore, blood pressure. The absolute bioavailability of cilazaprilat after oral administration of cilazapril is 57% based on urinary recovery data. (The absolute bioavailability of cilazaprilat after oral administration of cilazaprilat is 19%.) Ingestion of food immediately before the administration of cilazapril reduces the average peak plasma concentration of cilazaprilat by 29%, delays the peak by one hour and reduces the bioavailability of cilazaprilat by 14%. These pharmacokinetic changes have little influence on plasma ACE inhibition.
Angiotensin-Converting Enzyme Inhibitors
A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. (See all compounds classified as Angiotensin-Converting Enzyme Inhibitors.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
C09AA08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C09 - Agents acting on the renin-angiotensin system
C09A - Ace inhibitors, plain
C09AA - Ace inhibitors, plain
C09AA08 - Cilazapril
Absorption
Maximum plasma concentrations of cilazaprilat are reached within two hours after administration of cilazapril.
Route of Elimination
Cilazaprilat is eliminated unchanged by the kidneys. The total urinary recovery of cilazaprilat after intravenous administration of 2.5 mg is 91%.
Clearance
Total clearance is 12.3 L/h and renal clearance is 10.8 L/h. The total urinary recovery of cilazaprilat following the oral administration of 2.5 mg cilazapril is 52.6%.
Half-lives for the periods 1 to 4 hours and 1 to 7 days after the intravenous administration of 2.5 mg cilazaprilat are 0.90 and 46.2 hours respectively.
Cilazapril is a pyridazine ACE inhibitor. It competes with angiotensin I for binding at the angiotensin-converting enzyme, blocking the conversion of angiotensin I to angiotensin II. As angiotensin II is a vasoconstrictor and a negative feedback mediator for renin activity, lower angiotensin II levels results in a decrease in blood pressure, an increase in renin activity, and stimulation of baroreceptor reflex mechanisms. Kininase II, an enzyme which degrades the vasodilator bradykinin, is identical to ACE and may also be inhibited.


