



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
EU WC
0
Listed Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0

1. Alpha-chymotrypsin Choay
2. Alphacutane
3. Avazyme
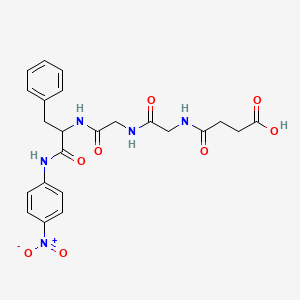
1. Chymotrypsin Substrate I, Colorimetric
2. 9004-07-3
3. 4-[[2-[[2-[[1-(4-nitroanilino)-1-oxo-3-phenylpropan-2-yl]amino]-2-oxoethyl]amino]-2-oxoethyl]amino]-4-oxobutanoic Acid
4. A-chymotrypsin
5. Dtxsid30988741
6. Akos024350585
7. Db-055212
8. Ft-0641215
9. N-succinyl-gly-gly-phe-p-nitroanilide, Protease Substrate
10. N-(3-carboxy-1-oxopropyl)glycylglycyl-n-(4-nitrophenyl)-l-phenylalaninamide
11. 4-hydroxy-4-({2-hydroxy-2-[(2-hydroxy-2-{[1-(4-nitroanilino)-1-oxo-3-phenylpropan-2-yl]imino}ethyl)imino]ethyl}imino)butanoic Acid
1. Alpha Chymotrypsin
| Molecular Weight | 499.5 g/mol |
|---|---|
| Molecular Formula | C23H25N5O8 |
| XLogP3 | 0.4 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 12 |
| Exact Mass | 499.17031277 g/mol |
| Monoisotopic Mass | 499.17031277 g/mol |
| Topological Polar Surface Area | 200 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 801 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
EXPTL USE (MEDICATION (VET):): CHYMOTRYPSIN CAN BE SHOWN TO HAVE AN ANTI-INFLAMMATORY ACTION IN EXPTL ANIMALS, BUT ONLY WHEN THE ENZYME IS ADMIN PARENTERALLY IN DOSES 10 TO 20 TIMES THOSE EMPLOYED CLINICALLY & PRIOR TO THE PRODN OF THE INFLAMMATION.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 957
...USED FOR RELIEF OF SYMPTOMS RELATED TO EPISIOTOMY. ITS USEFULNESS IN INFLAMMATORY STATES SECONDARY TO SURGICAL OR PHYSICAL TRAUMA REMAINS UNPROVEN.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 957
ALPHA-CHYMOTRYPSIN...IS...USED IN CATARACT OPERATIONS TO LOOSEN THE LENS AFTER INCISION OF CORNEA. AFTER THE CORNEOSCLERAL OR CORNEOSCLERALCONJUNCTIVAL INCISION, THE POSTERIOR CHAMBER IS IRRIGATED WITH ABOUT 2 ML OF ENZYME SOLN (150 UNITS PER ML) TO FRAGMENT FIBERS OF THE ZONULE (ENZYMATIC ZONULOLYSIS).
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 957
...FOR DEBRIDEMENT OF NECROTIC WOUNDS, ULCERS, ABSCESSES, EMPYEMAS, & FISTULAS. IT HAS BEEN USED ALSO FOR LIQUIFACTION OF BLOOD & EXUDATES THAT HAVE NOT BECOME ORGANIZED BY FIBROUS TISSUE.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 972
For more Therapeutic Uses (Complete) data for CHYMOTRYPSIN (10 total), please visit the HSDB record page.
...IT IS NOT RECOMMENDED FOR USE IN OPHTHALMIC SURGERY IN PT UNDER 20 YEARS OF AGE BECAUSE OF POSSIBLE LOSS OF VITREOUS HUMOR.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 957
GLAUCOMA, AS A COMPLICATION OF USE OF CHYMOTRYPSIN IN CATARACT EXTRACTIONS IN PT, HAS OCCURED USUALLY WITHIN 2 TO 5 DAYS AFTER OPERATION... THERE HAVE BEEN PRACTICALLY NO INDICATIONS OF LONGER-PERSISTING GLAUCOMA, FROM USE OF THE ENZYME.
Grant, W. M. Toxicology of the Eye. 2nd ed. Springfield, Illinois: Charles C. Thomas, 1974., p. 292
No therapeutic indications.
Chymotrypsin is a digestive enzyme synthesized in the pancreas that plays an essential role in proteolysis, or the breakdown of proteins and polypeptides. As a component in the pancreatic juice, chymotrypsin aids in the digestion of proteins in the duodenum by preferentially cleaving peptide amide bonds.
B - Blood and blood forming organs
B06 - Other hematological agents
B06A - Other hematological agents
B06AA - Enzymes
B06AA04 - Chymotrypsin
S - Sensory organs
S01 - Ophthalmologicals
S01K - Surgical aids
S01KX - Other surgical aids
S01KX01 - Chymotrypsin
Absorption
No pharmacokinetic data available.
Route of Elimination
No pharmacokinetic data available.
Volume of Distribution
No pharmacokinetic data available.
Clearance
No pharmacokinetic data available.
No pharmacokinetic data available.
No pharmacokinetic data available.
Chymotrypsin is synthesized by pancreatic acinar cells as an inactive precursor, chymotrypsinogen, that is secreted to the duodenum and activated via trypsin-induced cleavage. It also induces its own activation by cleaving essential amino acid residues in the oxyanion hole to produce -Chymotrypsin, which is a more stable form than -Chymotrypsin. Residues His-57, Asp-102, and Ser-195 form the catalytic triad while residues 189195, 214220, and 225228 form the primary substrate-binding pocket called S1 binding pocket. Residue 189 in the polar serine residue that lies at the bottom of the S1 binding pocket. Chymotrypsin favors aromatic residues like phenylalanine, tyrosine, and tryptophan but may hydrolyze other bonds in peptides at slower rates.
THEY SPLIT SECONDARY AMIDE OR PEPTIDE BONDS, CARBOXYLIC OR PHENOLIC ESTER BONDS & EVEN CARBON-CARBON BONDS. THEIR MAIN FUNCTION IS TO HYDROLYZE PEPTIDE BONDS DURING THE INTESTINAL DIGESTION OF PROTEINS. /CHYMOTRYPSINS/
The Merck Index. 9th ed. Rahway, New Jersey: Merck & Co., Inc., 1976., p. 293


