
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
Canada
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
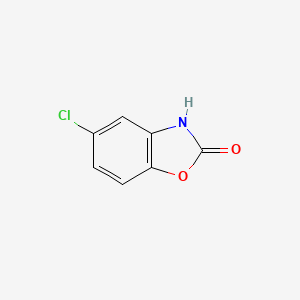

1. Paraflex
2. Parafon
3. Parafon Forte Dsc
1. 95-25-0
2. Paraflex
3. Chloroxazone
4. Chlorzoxazon
5. 5-chloro-2-benzoxazolone
6. 5-chloro-2-benzoxazolinone
7. 2(3h)-benzoxazolone, 5-chloro-
8. Biomioran
9. Escoflex
10. Myoflexin
11. Myoflexine
12. Pathorysin
13. 5-chloro-2-hydroxybenzoxazole
14. Mioran
15. Miotran
16. Neoflex
17. Solaxin
18. 5-chloro-2(3h)-benzoxazolone
19. Parafon Forte Dsc
20. 5-chlorobenzoxazolidone
21. 5-chlorobenzo[d]oxazol-2(3h)-one
22. 5-chloro-2-benzoxazolol
23. Chlorzoxane
24. 5-chloro-1,3-benzoxazol-2(3h)-one
25. Usaf Ma-10
26. 5-chlorobenzoxazol-2-one
27. 5-chloro-3h-1,3-benzoxazol-2-one
28. Strifon Forte Dsc
29. Parafon
30. 5-chlorobenzoxazolone
31. 2-hydroxy-5-chlorobenzoxazole
32. Chlorzoxazonum
33. 5-chlorbenzoxazolin-2-on
34. 5-chloro-3(h)-2-benzoxazolone
35. 5-chlorobenzo[d]oxazol-2-ol
36. 5-chlorobenzoksazolon-2
37. 5-chlorobenzoksazolinon-2
38. 5-chlorobenzoxazolin-2-one
39. Strifon
40. Nsc 26189
41. 2-benzoxazolinone, 5-chloro-
42. 5-chloro-3h-benzooxazol-2-one
43. 5-chloro-1,3-benzoxazol-2-ol
44. Chembl1371
45. Mls000069380
46. Chebi:3655
47. H0de420u8g
48. Chlorsoxazone
49. Klorzoxazon
50. Nyoflex
51. Remofleks
52. 5-chloro-2,3-dihydro-1,3-benzoxazol-2-one
53. Parafon Forte
54. Nsc-26189
55. Cas-95-25-0
56. Ncgc00015238-02
57. Clorzoxazona
58. Lorzone
59. Smr000058269
60. Dsstox_cid_2813
61. Dsstox_rid_76739
62. Dsstox_gsid_22813
63. Clw
64. Chlorzoxazonum [inn-latin]
65. Clorzoxazona [inn-spanish]
66. Chlorzoxazona
67. 5-chlorobenzoksazolon-2 [polish]
68. 5-chlorobenzoksazolinon-2 [polish]
69. Paraflex (tn)
70. Sr-01000075207
71. Einecs 202-403-9
72. Mfcd00005717
73. Component Of Parafon Forte
74. Unii-h0de420u8g
75. Ai3-63119
76. Chlorzoxazone (jan/usp/inn)
77. Prestwick_62
78. Chlorzoxazone,(s)
79. Chlorzoxazone [usp:inn:ban:jan]
80. 5-chlorobenzoxazolinone
81. Spectrum_000148
82. Opera_id_1659
83. Prestwick0_000163
84. Prestwick1_000163
85. Prestwick2_000163
86. Prestwick3_000163
87. Spectrum2_001149
88. Spectrum3_000350
89. Spectrum4_000287
90. Spectrum5_000745
91. Lopac-c-4397
92. Chlorzoxazone [mi]
93. C 4397
94. Chlorzoxazone [inn]
95. Chlorzoxazone [jan]
96. 5-chloro-benzooxazol-2-ol
97. 5-chloro-benzoxazolin-2-one
98. Lopac0_000253
99. Schembl35177
100. Bspbio_000025
101. Bspbio_002019
102. Chlorzoxazone [vandf]
103. Kbiogr_000814
104. Kbioss_000628
105. Wln: T56 Bmvoj Hg
106. Chlorzoxazone [mart.]
107. Divk1c_000895
108. Spectrum1500188
109. Spbio_001077
110. Spbio_001946
111. Chlorzoxazone [usp-rs]
112. Chlorzoxazone [who-dd]
113. Bpbio1_000029
114. Gtpl2322
115. Dtxsid9022813
116. Hms502m17
117. Kbio1_000895
118. Kbio2_000628
119. Kbio2_003196
120. Kbio2_005764
121. Kbio3_001239
122. Ninds_000895
123. Chlorzoxazone-[13c,15n,18o]
124. Hms1568b07
125. Hms1920o07
126. Hms2091e14
127. Hms2095b07
128. Hms2235i19
129. Hms3259i15
130. Hms3260d08
131. Hms3373p17
132. Hms3652k22
133. Hms3712b07
134. Hms3885n07
135. Pharmakon1600-01500188
136. 5-chloro-2-hydroxybenzo[d]oxazole
137. Chlorzoxazone [orange Book]
138. Act08248
139. Albb-012641
140. Bcp07916
141. Hy-b1462
142. Nsc26189
143. Str00805
144. Chlorzoxazone [usp Impurity]
145. Tox21_110105
146. Tox21_500253
147. Bdbm50290811
148. Ccg-40323
149. Chlorzoxazone [usp Monograph]
150. Nsc756693
151. S4155
152. Stk071582
153. Zinc84843283
154. Akos000404381
155. Tox21_110105_1
156. Component Of Parafon Forte (salt/mix)
157. Cs-5155
158. Db00356
159. Hg-0202
160. Lp00253
161. Nc00499
162. Nsc-756693
163. Sdccgsbi-0050241.p005
164. 5-chloranyl-3h-1,3-benzoxazol-2-one
165. 5-chloro-1,3-benzoxazole-2(3h)-one
166. Idi1_000895
167. Chlorzoxazone 1.0 Mg/ml In Acetonitrile
168. Ncgc00015238-01
169. Ncgc00015238-03
170. Ncgc00015238-04
171. Ncgc00015238-05
172. Ncgc00015238-06
173. Ncgc00015238-07
174. Ncgc00015238-08
175. Ncgc00015238-09
176. Ncgc00015238-10
177. Ncgc00015238-13
178. Ncgc00015238-24
179. Ncgc00093714-01
180. Ncgc00093714-02
181. Ncgc00093714-03
182. Ncgc00093714-04
183. Ncgc00260938-01
184. 5-chloro-1,3-benzoxazol-2(3h)-one #
185. Ac-12192
186. Bp-11613
187. Meso-butane-1,2,3,4-tetracarboxylicacid
188. Sbi-0050241.p004
189. Db-057571
190. Ab00051947
191. Eu-0100253
192. Ft-0620220
193. Sw196377-3
194. En300-31026
195. A16449
196. C07931
197. D00771
198. D78096
199. Ab00051947_05
200. Ab00051947_06
201. A845253
202. Q3294630
203. Sr-01000075207-1
204. Sr-01000075207-3
205. Sr-01000075207-5
206. W-100168
207. Brd-k98174813-001-05-7
208. Brd-k98174813-001-08-1
209. Z57183224
210. Chlorzoxazone, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 169.56 g/mol |
|---|---|
| Molecular Formula | C7H4ClNO2 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 168.9930561 g/mol |
| Monoisotopic Mass | 168.9930561 g/mol |
| Topological Polar Surface Area | 38.3 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 185 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Chlorzoxazone |
| PubMed Health | Chlorzoxazone (By mouth) |
| Drug Classes | Skeletal Muscle Relaxant, Centrally Acting |
| Drug Label | Chlorzoxazone USP is a centrally acting skeletal muscle relaxant, available as tablets of 500 mg for oral administration. Its chemical name is 5-Chloro-2-benzoxazolinone, and its structural formula is: Chlorzoxazone Structural FormulaC7H4CINO2 MW 1... |
| Active Ingredient | Chlorzoxazone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 750mg; 375mg; 500mg |
| Market Status | Prescription |
| Company | Watson Labs; Mikart; Barr |
| 2 of 4 | |
|---|---|
| Drug Name | Parafon forte dsc |
| PubMed Health | Chlorzoxazone (By mouth) |
| Drug Classes | Skeletal Muscle Relaxant, Centrally Acting |
| Drug Label | Each caplet (capsule shaped tablet) contains:*5-chlorobenzoxazolinoneChlorzoxazone*500 mgInactive ingredients: FD&C Blue No. 1, microcrystalline cellulose, docusate sodium, lactose (hydrous), magnesium stearate, sodium benzoate, sodium star... |
| Active Ingredient | Chlorzoxazone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Janssen R And D |
| 3 of 4 | |
|---|---|
| Drug Name | Chlorzoxazone |
| PubMed Health | Chlorzoxazone (By mouth) |
| Drug Classes | Skeletal Muscle Relaxant, Centrally Acting |
| Drug Label | Chlorzoxazone USP is a centrally acting skeletal muscle relaxant, available as tablets of 500 mg for oral administration. Its chemical name is 5-Chloro-2-benzoxazolinone, and its structural formula is: Chlorzoxazone Structural FormulaC7H4CINO2 MW 1... |
| Active Ingredient | Chlorzoxazone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 750mg; 375mg; 500mg |
| Market Status | Prescription |
| Company | Watson Labs; Mikart; Barr |
| 4 of 4 | |
|---|---|
| Drug Name | Parafon forte dsc |
| PubMed Health | Chlorzoxazone (By mouth) |
| Drug Classes | Skeletal Muscle Relaxant, Centrally Acting |
| Drug Label | Each caplet (capsule shaped tablet) contains:*5-chlorobenzoxazolinoneChlorzoxazone*500 mgInactive ingredients: FD&C Blue No. 1, microcrystalline cellulose, docusate sodium, lactose (hydrous), magnesium stearate, sodium benzoate, sodium star... |
| Active Ingredient | Chlorzoxazone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Janssen R And D |
For the relief of discomfort associated with acute painful musculoskeletal conditions.
Chlorzoxazone is a centrally-acting agent for painful musculoskeletal conditions. Data available from animal experiments as well as human study indicate that chlorzoxazone acts primarily at the level of the spinal cord and subcortical areas of the brain where it inhibits multisynaptic reflex a.c. involved in producing and maintaining skeletal muscle spasm of varied etiology. The clinical result is a reduction of the skeletal muscle spasm with relief of pain and increased mobility of the involved muscles.
Muscle Relaxants, Central
A heterogeneous group of drugs used to produce muscle relaxation, excepting the neuromuscular blocking agents. They have their primary clinical and therapeutic uses in the treatment of muscle spasm and immobility associated with strains, sprains, and injuries of the back and, to a lesser degree, injuries to the neck. They have been used also for the treatment of a variety of clinical conditions that have in common only the presence of skeletal muscle hyperactivity, for example, the muscle spasms that can occur in MULTIPLE SCLEROSIS. (From Smith and Reynard, Textbook of Pharmacology, 1991, p358) (See all compounds classified as Muscle Relaxants, Central.)
M - Musculo-skeletal system
M03 - Muscle relaxants
M03B - Muscle relaxants, centrally acting agents
M03BB - Oxazol, thiazine, and triazine derivatives
M03BB03 - Chlorzoxazone
Route of Elimination
Chlorzoxazone is rapidly metabolized and is excreted in the urine, primarily in a conjugated form as the glucuronide.
Chlorzoxazone is rapidly metabolized in the liver and is excreted in the urine, primarily in a conjugated form as the glucuronide.
Chlorzoxazone has known human metabolites that include 6-Hydroxychlorzoxazone.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Chlorzoxazone inhibits degranulation of mast cells, subsequently preventing the release of histamine and slow-reacting substance of anaphylaxis (SRS-A), mediators of type I allergic reactions. Chlorzoxazone also may reduce the release of inflammatory leukotrienes. Chlorzoxazone may act by inhibiting calcium and potassium influx which would lead to neuronal inhibition and muscle relaxation. Data available from animal experiments as well as human study indicate that chlorzoxazone acts primarily at the level of the spinal cord and subcortical areas of the brain where it inhibits multisynaptic reflex arcs involved in producing and maintaining skeletal muscle spasm

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
100
PharmaCompass offers a list of Chlorzoxazone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Chlorzoxazone manufacturer or Chlorzoxazone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Chlorzoxazone manufacturer or Chlorzoxazone supplier.
PharmaCompass also assists you with knowing the Chlorzoxazone API Price utilized in the formulation of products. Chlorzoxazone API Price is not always fixed or binding as the Chlorzoxazone Price is obtained through a variety of data sources. The Chlorzoxazone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Chlorzoxazone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Chlorzoxazone, including repackagers and relabelers. The FDA regulates Chlorzoxazone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Chlorzoxazone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Chlorzoxazone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Chlorzoxazone supplier is an individual or a company that provides Chlorzoxazone active pharmaceutical ingredient (API) or Chlorzoxazone finished formulations upon request. The Chlorzoxazone suppliers may include Chlorzoxazone API manufacturers, exporters, distributors and traders.
click here to find a list of Chlorzoxazone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Chlorzoxazone DMF (Drug Master File) is a document detailing the whole manufacturing process of Chlorzoxazone active pharmaceutical ingredient (API) in detail. Different forms of Chlorzoxazone DMFs exist exist since differing nations have different regulations, such as Chlorzoxazone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Chlorzoxazone DMF submitted to regulatory agencies in the US is known as a USDMF. Chlorzoxazone USDMF includes data on Chlorzoxazone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Chlorzoxazone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Chlorzoxazone suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Chlorzoxazone Drug Master File in Korea (Chlorzoxazone KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Chlorzoxazone. The MFDS reviews the Chlorzoxazone KDMF as part of the drug registration process and uses the information provided in the Chlorzoxazone KDMF to evaluate the safety and efficacy of the drug.
After submitting a Chlorzoxazone KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Chlorzoxazone API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Chlorzoxazone suppliers with KDMF on PharmaCompass.
A Chlorzoxazone written confirmation (Chlorzoxazone WC) is an official document issued by a regulatory agency to a Chlorzoxazone manufacturer, verifying that the manufacturing facility of a Chlorzoxazone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Chlorzoxazone APIs or Chlorzoxazone finished pharmaceutical products to another nation, regulatory agencies frequently require a Chlorzoxazone WC (written confirmation) as part of the regulatory process.
click here to find a list of Chlorzoxazone suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Chlorzoxazone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Chlorzoxazone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Chlorzoxazone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Chlorzoxazone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Chlorzoxazone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Chlorzoxazone suppliers with NDC on PharmaCompass.
Chlorzoxazone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Chlorzoxazone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Chlorzoxazone GMP manufacturer or Chlorzoxazone GMP API supplier for your needs.
A Chlorzoxazone CoA (Certificate of Analysis) is a formal document that attests to Chlorzoxazone's compliance with Chlorzoxazone specifications and serves as a tool for batch-level quality control.
Chlorzoxazone CoA mostly includes findings from lab analyses of a specific batch. For each Chlorzoxazone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Chlorzoxazone may be tested according to a variety of international standards, such as European Pharmacopoeia (Chlorzoxazone EP), Chlorzoxazone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Chlorzoxazone USP).

