



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
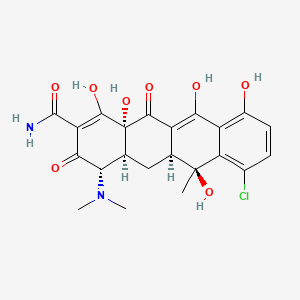

1. 4-epimer Chlortetracycline
2. Aureocyclin
3. Aureomycin
4. Aureomycine
5. Biomycin
6. Bisulfate, Chlortetracycline
7. Calcium Salt Chlortetracycline
8. Chlorotetracycline
9. Chlortetracycline Bisulfate
10. Chlortetracycline Hydrochloride
11. Chlortetracycline Monohydrochloride
12. Chlortetracycline Sulfate (1:1)
13. Chlortetracycline Sulfate (2:1)
14. Chlortetracycline, 4 Epimer
15. Chlortetracycline, 4-epimer
16. Chlortetracycline, Calcium Salt
17. Hydrochloride, Chlortetracycline
18. Monohydrochloride, Chlortetracycline
19. Salt Chlortetracycline, Calcium
1. 57-62-5
2. 7-chlorotetracycline
3. Chlorotetracycline
4. Aureomycin
5. Acronize
6. Chlormax
7. Clortetraciclina
8. Chlortetracyclinum
9. Aureomycin A-377
10. Biomycin
11. (4s,4as,5as,6s,12as)-7-chloro-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide
12. Duomycin
13. Wck1kiq23q
14. Aureomykoin
15. Chrysomykine
16. Aureocina
17. Biomitsin
18. Flamycin
19. Uromycin
20. Biomycin A
21. Chebi:27644
22. Ctc (abtibiotic)
23. Tri-chlortetracycline
24. Aureomycin (tn)
25. Chlortetracycline (inn)
26. Chlortetracycline [inn]
27. Caswell No. 219b
28. Unii-wck1kiq23q
29. Aueromycin
30. Chlortetracycline [inn:ban]
31. Chlortetracyclinum [inn-latin]
32. Clortetraciclina [inn-spanish]
33. 2-naphthacenecarboxamide, 7-chloro-4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, (4s,4as,5as,6s,12as)-
34. Einecs 200-341-7
35. Mfcd00864876
36. Epa Pesticide Chemical Code 006301
37. Spectrum_000727
38. Spectrum_001315
39. Prestwick0_000145
40. Prestwick1_000145
41. Prestwick2_000145
42. Spectrum2_000802
43. Spectrum3_000348
44. Spectrum4_001001
45. Spectrum4_001791
46. Spectrum5_000461
47. Spectrum5_001870
48. Pig Liver-chlortetracycline
49. Schembl3110
50. Pig Kidney (ctc Free)
51. Pig Muscle (ctc Free)
52. Schembl62861
53. Bspbio_002015
54. Kbiogr_001461
55. Kbiogr_002364
56. Kbioss_001207
57. Kbioss_001795
58. Chlortetracycline [mi]
59. Divk1c_000253
60. Spbio_000663
61. Spbio_002189
62. Chembl404520
63. Schembl4099094
64. Chembl1622557
65. Dtxsid9022811
66. Schembl19981439
67. Chlortetracycline [vandf]
68. Hms500m15
69. Hy-b1327a
70. Kbio1_000253
71. Kbio2_001207
72. Kbio2_001795
73. Kbio2_003775
74. Kbio2_004363
75. Kbio2_006343
76. Kbio2_006931
77. Kbio3_001235
78. Chlortetracycline [mart.]
79. Ninds_000253
80. Chlortetracycline [who-dd]
81. (4s,4as,5as,6s,12ar)-7-chloro-4-(dimethylamino)-1,6,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide
82. Rkl10090
83. Lmpk07000004
84. Zinc19701769
85. Chlortetracycline [green Book]
86. Zinc100154659
87. Zinc100302624
88. Ccg-269531
89. Chlortetracycline [ep Impurity]
90. Db09093
91. Idi1_000253
92. Ncgc00178862-01
93. Ncgc00178862-04
94. E702
95. Sbi-0051312.p003
96. Cs-0013541
97. Lymecycline Impurity G [ep Impurity]
98. C06571
99. D07689
100. H10649
101. Ab00643434_03
102. 864c876
103. Q417948
104. Q-200843
105. (4s,5as,6s,12as)-7-chloro-4-(dimethylamino)-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide
| Molecular Weight | 478.9 g/mol |
|---|---|
| Molecular Formula | C22H23ClN2O8 |
| XLogP3 | -1.3 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 2 |
| Exact Mass | 478.1142934 g/mol |
| Monoisotopic Mass | 478.1142934 g/mol |
| Topological Polar Surface Area | 182 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 1010 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used in the manufacuring of medicated animal feeds.
FDA Label
Tetracycline antibiotics are bacteriostatic agents which act to inhibit bacterial growth and reproduction.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Antiprotozoal Agents
Substances that are destructive to protozoans. (See all compounds classified as Antiprotozoal Agents.)
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)
S01AA02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A01 - Stomatological preparations
A01A - Stomatological preparations
A01AB - Antiinfectives and antiseptics for local oral treatment
A01AB21 - Chlortetracycline
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06A - Antibiotics for topical use
D06AA - Tetracycline and derivatives
D06AA02 - Chlortetracycline
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01A - Tetracyclines
J01AA - Tetracyclines
J01AA03 - Chlortetracycline
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AA - Antibiotics
S01AA02 - Chlortetracycline
Absorption
Chortetracycline reaches peak plasma concentation in about 3 hours. Its oral bioavailability is 25-30%.
Route of Elimination
Chlortetracycline is mainly eliminated in feces. Renal function does not appear to affect the rate of elimination.
Volume of Distribution
Chlortetracycline has a volume of distribution of 100 liters.
Chlortetracycline is not known to undergo significant metabolism.
The half-life of Chlortetracycline is 5.6 hours.
Chlortetracycline, like other tetracyclines, competes for the A site of the bacterial ribosome. This binding competes with tRNA carrying amino acids preventing the addition of more amino acids to the peptide chain. This inhibition of protein synthesis ultimately inhibits growth and reproduction of the bacterial cell as necessary proteins cannot be synthesized.


