



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
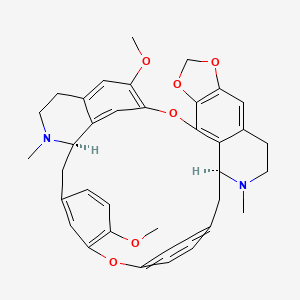

1. 6',12'-dimethoxy-2,2'-dimethyl-6,7-(methylenebis(oxy))oxyacanthan
2. Cepharanthin
1. 481-49-2
2. Cepharanthin
3. (+)-cepharanthine
4. O-methylcepharanoline
5. Cepharantin
6. Cepharanthine [jan]
7. 6',12'-dimethoxy-2,2'-dimethyl-6,7-(methylenebis(oxy))oxyacanthan
8. Nsc-623442
9. Chebi:3546
10. 7592yj0j6t
11. Nsc623442
12. Cepharanthine (jan)
13. (14s,27r)-22,33-dimethoxy-13,28-dimethyl-2,5,7,20-tetraoxa-13,28-diazaoctacyclo[25.6.2.216,19.13,10.121,25.04,8.031,35.014,39]nonatriaconta-1(33),3(39),4(8),9,16(38),17,19(37),21,23,25(36),31,34-dodecaene
14. Oxyacanthan, 6',12'-dimethoxy-2,2'-dimethyl-6,7-(methylenebis(oxy))-
15. Ecaene (non-preferred Name)
16. Dsstox_cid_25957
17. Dsstox_rid_81253
18. Dsstox_gsid_45957
19. 22,33-dimethoxy-13,28-dimethyl-2,5,7,20-tetraoxa-13,28-diazaoctacyclo[25.6.2.216,19.13,10.121,25.04,8.031,35.014,39]nonatriaconta-1(33),3(39),4(8),9,16(38),17,19(37),21,23,25(36),31,34-dodecaene
20. Cas-481-49-2
21. Ccris 6539
22. Sr-01000779734
23. Nsc 623442
24. Brn 0075231
25. Unii-7592yj0j6t
26. Cepharanthin,(s)
27. Ncgc00095194-01
28. (14s,27r)-22,33-dimethoxy-13,28-dimethyl-2,5,7,20-tetraoxa-13,28-diazaoctacyclo[25.6.2.2~16,19~.1~3,10~.1~21,25~.0~4,8~.0~14,39~.0~31,35~]nonatriaconta-1(33),3,8,10(39),16,18,21(36),22,24,31,34,37-dod
29. Cepharanthine (tn)
30. Spectrum2_000832
31. Spectrum3_001963
32. 12-o-methylcepharanoline
33. Cepharanthine [mi]
34. Upcmld-dp054
35. Bspbio_003563
36. 4-27-00-09061 (beilstein Handbook Reference)
37. Mls000728518
38. Schembl154545
39. Spectrum1505322
40. Spbio_000783
41. Cepharanthine [who-dd]
42. Chembl449782
43. Dtxsid6045957
44. Upcmld-dp054:001
45. Kbio3_002909
46. Cepharanthine, >=95% (hplc)
47. Cepharanthine, >=98% (hplc)
48. Hms1922j12
49. Hms2232f21
50. Pharmakon1600-01505322
51. Hy-n6972
52. Tox21_111483
53. Bbl030154
54. Bdbm50423643
55. Ccg-40294
56. Mfcd00210482
57. Nsc758965
58. S4238
59. Stk801907
60. Zinc30726863
61. Akos004119865
62. Tox21_111483_1
63. Nsc-758965
64. Sdccgmls-0066893.p001
65. Ncgc00161621-01
66. Ncgc00161621-02
67. Ncgc00161621-03
68. Ncgc00161621-05
69. Ncgc00161621-13
70. Ac-15206
71. As-17451
72. Smr000445632
73. Sbi-0207049.p001
74. Cs-0007138
75. M2968
76. C09391
77. D01035
78. Ab00643356_08
79. Ab00643356_09
80. 481c492
81. A871948
82. Q-100524
83. Sr-01000779734-3
84. Sr-01000779734-4
85. Brd-k96194081-001-06-0
86. Q15410888
87. (14s,27r)-22,33-dimethoxy-13,28-dimethyl-2,5,7,20-tetraoxa-13,28-diazaoctacyclo[25.6.2.2(16,19).1(3,10).1(21,25).0(4,8).0(14,39).0(31,35)]nonatriaconta-1(33),3,8,10(39),16,18,21(36),22,24,31,34,37-dodecaene
88. (14s,27r)-22,33-dimethoxy-13,28-dimethyl-2,5,7,20-tetraoxa-13,28-diazaoctacyclo[25.6.2.2??,??.1?,??.1??,??.0?,?.0??,??.0??,??]nonatriaconta-1(33),3,8,10(39),16,18,21(36),22,24,31,34,37-dodecaene
| Molecular Weight | 606.7 g/mol |
|---|---|
| Molecular Formula | C37H38N2O6 |
| XLogP3 | 6.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 2 |
| Exact Mass | 606.27298694 g/mol |
| Monoisotopic Mass | 606.27298694 g/mol |
| Topological Polar Surface Area | 61.9 Ų |
| Heavy Atom Count | 45 |
| Formal Charge | 0 |
| Complexity | 994 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Radiation-Protective Agents
Drugs used to protect against ionizing radiation. They are usually of interest for use in radiation therapy but have been considered for other purposes, e.g. military. (See all compounds classified as Radiation-Protective Agents.)
Antineoplastic Agents, Phytogenic
Agents obtained from higher plants that have demonstrable cytostatic or antineoplastic activity. (See all compounds classified as Antineoplastic Agents, Phytogenic.)


