



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0

1. Bl P 1322
2. Bl-p 1322
3. Blp 1322
4. Brisfirina
5. Cfaloject
6. Cefadyl
7. Cefapirin
8. Cephapirin Monosodium Salt
9. Cephapirin Sodium
10. Cephapirin, Sodium
11. Monosodium Salt, Cephapirin
12. Salt, Cephapirin Monosodium
13. Sodium Cephapirin
1. Cefapirin
2. Cephapirine
3. Cefaprin
4. 21593-23-7
5. Cefadyl
6. Cefapirina
7. Cefapirine
8. Cefapirinum
9. Cepr
10. Cefapirine [inn-french]
11. Cefapirinum [inn-latin]
12. Cefapirina [inn-spanish]
13. Cefatrexyl
14. Ambrocef
15. Cefatrex
16. Chebi:554446
17. 7-(2-(4-pyridylthio)acetamido)cephalosporanic Acid
18. Cefa
19. 89b59h32vn
20. Cefaprin Sodium
21. (6r,7r)-3-(acetoxymethyl)-8-oxo-7-(2-(pyridin-4-ylthio)acetamido)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
22. (6r,7r)-3-(acetyloxymethyl)-8-oxo-7-[(2-pyridin-4-ylsulfanylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
23. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 3-((acetyloxy)methyl)-8-oxo-7-(((4-pyridinylthio)acetyl)amino)-, (6r-trans)-
24. Cefapirin [inn:ban]
25. Antibiotic Bl-p1322
26. Cefapirin (ban)
27. Metricure (tn)
28. (6r,7r)-3-(acetoxymethyl)-8-oxo-7-{[(pyridin-4-ylsulfanyl)acetyl]amino}-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
29. 24356-60-3
30. Cefapilin
31. 356c603
32. Cefa-lak
33. Hsdb 3215
34. Cefa -ak
35. Einecs 244-466-5
36. Brn 1095157
37. Nsc179171
38. Spectrum_000112
39. Cefapirin [inn]
40. Cephapirin [mi]
41. Cefapirin [hsdb]
42. Prestwick0_000851
43. Prestwick1_000851
44. Prestwick2_000851
45. Spectrum2_000103
46. Spectrum3_000333
47. Spectrum4_000270
48. Spectrum5_000671
49. Cephapirin [vandf]
50. Epitope Id:116226
51. Cefapirin [who-dd]
52. Schembl3205
53. Chembl1599
54. Lopac0_000279
55. Bspbio_001965
56. Kbiogr_000740
57. Kbioss_000552
58. (6r,7r)-3-(acetoxymethyl)-8-oxo-7-(2-(4-pyridylthio)acetamido)-5-thia-1-azabicyclo(4.2.0)oct-2-en-2-carbonsaeure
59. 3-(hydroxymethyl)-8-oxo-7-(2-(4-pyridylthio)acetamidol-5-thia-1-azabicyclo(4.2.0)oct-2-en-2carbonsaeure Acetat
60. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 3-(hydroxymethyl)-8-oxo-7-(2-(4-pyridylthio)acetamido)-, Acetate (ester)
61. Divk1c_000042
62. Unii-89b59h32vn
63. Spbio_000086
64. Spbio_002782
65. Cephalosporanic Acid, 7-(2-(4-pyridylthio)acetamido)-
66. Dtxsid9022784
67. Gtpl12191
68. Kbio1_000042
69. Kbio2_000552
70. Kbio2_003120
71. Kbio2_005688
72. Kbio3_001185
73. Ninds_000042
74. Hy-a0153
75. Zinc3830511
76. Bdbm50370592
77. Akos015896499
78. Db01139
79. Idi1_000042
80. (6r,7r)-3-[(acetyloxy)methyl]-8-oxo-7-{[(pyridin-4-ylthio)acetyl]amino}-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
81. (6r,7r)-3-acetoxymethyl-7-[(pyridin-4-ylsulfanyl)acetamido]-3,4-didehydrocepham-4-carboxylic Acid
82. Sbi-0050267.p004
83. Cs-0017478
84. C06896
85. D07636
86. Q549803
87. J-014163
88. 7-(.alpha.-(4-pyridylthio)acetamido)cephalosporanic Acid
89. (6r,7r)-3-(acetoxymethyl)-8-oxo-7-(2-(pyridin-4-ylthio)acetamido)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylicacid
90. (6r,7r)-3-(acetoxymethyl)-8-oxo-7-[[2-(4-pyridylsulfanyl)acetyl]amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
91. (6r,7r)-3-(hydroxymethyl)-8-oxo-7-(2-(4-pyridylthio)acetamido)-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylate Acetate (ester)
92. (6r,7r)-3-[(acetyloxy)methyl]-8-oxo-7-[2-(pyridin-4-ylsulfanyl)acetamido]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
93. 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid, 3-[(acetyloxy)methyl]-8-oxo-7-[[(4-pyridinylthio)acetyl]amino]-, (6r,7r)-
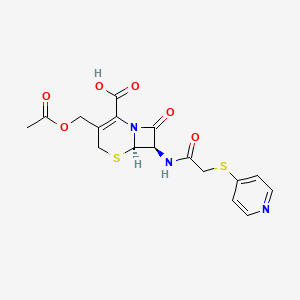
| Molecular Weight | 423.5 g/mol |
|---|---|
| Molecular Formula | C17H17N3O6S2 |
| XLogP3 | -1.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 8 |
| Exact Mass | 423.05587762 g/mol |
| Monoisotopic Mass | 423.05587762 g/mol |
| Topological Polar Surface Area | 177 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 707 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cephalosporins
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Cephalosporins are still useful as alternatives to penicillins for a variety of infections in patients who can't tolerate penicillins. These include streptococcal and staphylococcal infections.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1091
BACTERIA SUSCEPTIBLE TO CEPHALOTHIN /BOTH GRAM POSITIVE & GRAM-NEGATIVE MICROORGANISMS/ ARE SENSITIVE OVER APPROX SAME RANGE OF CONCN TO ... CEPHAPIRIN. ... CEPHAPIRIN IS SOMEWHAT MORE INHIBITORY THAN CEPHALOTHIN FOR GROUP-A STREP PYOGENES & PNEUMOCOCCUS.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1160
CEPHAPIRIN IS ONE OF NEWEST SEMISYNTHETIC CEPHALOSPORINS. ... LIKE OTHER CEPHALOSPORINS, CEPHAPIRIN SHOULD BE RESERVED FOR THOSE INSTANCES IN WHICH ORGANISM IS SENSITIVE TO IT & PATIENT IS HYPERSENSITIVE TO PENICILLIN.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 719
For more Therapeutic Uses (Complete) data for CEPHAPIRIN (10 total), please visit the HSDB record page.
SINCE ... CEPHAPIRIN ... ADMIN AS ... SODIUM SALTS, CARE SHOULD BE EXERCISED IN USE OF LARGE DOSES IN PERSONS WITH IMPAIRED CAPACITY TO EXCRETE THIS CATION. ... SUPRAINFECTIONS, USUALLY DUE TO GRAM NEGATIVE BACTERIA, MAY OCCUR WHEN THESE ANTIBIOTICS ARE EMPLOYED.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1163
TYPICAL DOSAGE SCHEDULES ... FOR MOST OF CEPHALOSPORINS MUST BE MODIFIED FOR PATIENTS WITH IMPAIRED RENAL FUNCTION. /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1162
... HYPERSENSITIVITY REACTIONS TO CEPHALOSPORINS IS HIGHER IN PATIENTS WHO HAVE SHOWN ALLERGIC MANIFESTATIONS FOLLOWING ADMIN OF PENICILLIN. THIS APPEARS TO BE RELATED TO SENSITIZATION TO BETA-LACTAM RING COMMON TO BOTH THESE DRUGS. /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1163
... ENTEROCOCCAL ENDOCARDITIS CANNOT BE CURED WITH CEPHALOSPORIN EVEN WHEN ... GIVEN CONCURRENTLY WITH GENTAMICIN OR STREPTOMYCIN. ... ENTEROBACTER (AEROBACTER) INFECTIONS ARE, AS A RULE, RESISTANT TO THESE CMPD. /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1163
For more Drug Warnings (Complete) data for CEPHAPIRIN (10 total), please visit the HSDB record page.
For treatment of infections caused by susceptible bacteria.
Cephapirin is a first-generation cephalosporin that has a wide spectrum of activity against gram-positive and gram-negative organisms. Cephapirin is more resistant to beta-lactamases than are the penicillins and so is effective against staphylococci, with the exception of methicillin-resistant staphylococci.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DB - First-generation cephalosporins
J01DB08 - Cefapirin
... PENETRATION OF CEPHALOSPORINS INTO /CEREBROSPINAL FLUID/ IS POOR. /CEPHALOSPORINS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1164
CLOSE TO 50% OF CEPHAPIRIN IS BOUND TO PLASMA PROTEIN. HALF-LIFE ... IN NORMAL INDIVIDUALS IS ABOUT 40 MIN & IS DEPENDENT ON RENAL FUNCTION. SIGNIFICANT AMT ... PRESENT IN BLOOD IS REMOVED BY HEMODIALYSIS. ... EXCRETED MAINLY BY KIDNEY; ONLY 1% PRESENT IN BILE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
... /CEPHAPIRIN/ NOT ABSORBED FROM GI TRACT. IM INJECTION OF 0.5 G OF CEPHAPIRIN PRODUCES MAX PLASMA CONCN OF ABOUT 10 UG/ML @ 45 MIN; 1 G ABOUT 16 UG/ML. PLASMA CONCN ... EFFECTIVE AGAINST MANY SENSITIVE MICROORGANISMS ARE STILL DETECTABLE 6 HR AFTER SINGLE IM DOSE OF 1 G.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
ABOUT 30% OF IM DOSE OF CEPHAPIRIN IS EXCRETED IN URINE IN EACH OF 1ST 2 HR PERIODS AFTER INJECTION.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
For more Absorption, Distribution and Excretion (Complete) data for CEPHAPIRIN (8 total), please visit the HSDB record page.
Major metabolite detected is desacetylcephapirin.
MAJOR METABOLITE OF CEPHAPIRIN IS DEACETYLCEPHAPIRIN, WHICH IS ABOUT ONE HALF OF ANTIMICROBIAL ACTIVITY OF PARENT CMPD; 20% OF ANTIBIOTIC ACTIVITY IN PLASMA IS DUE TO DEACETYLATED CMPD.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1161
Cephapirin is partially metabolized in the plasma, liver and kidneys to diacetyl cephapirin, which has about 50% of the antibacterial activity of the parent cmpd. ... Up to 20% of the antibiotic activity in serum is due to the desacetyl metabolite.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 157
EIGHT HEALTHY ADULTS RECEIVED 1 G, IV AND IM. BIOLOGICAL HALF-LIFE OF CEPHAPIRIN WAS 43 MIN. ABSORPTION HALF-LIFE OF CEPHAPIRIN FROM IM ADMIN WAS 1.25 HR.
PMID:1206478 CABANA BE ET AL; J PHARMACOKINET BIOPHARM 3: 419-38 (1975)
IV SODIUM CEPHAPIRIN SHOWED BIEXPONENTIAL DISPOSITION CHARACTERISTICS IN SERUM & SUBCHONDRAL BONE, REACHING DISTRIBUTION EQUIL WITHIN 20 MIN. RAPIDLY ELIMINATED, WITH BETA HALF-LIFE OF ABOUT 0.3 HR & BODY CLEARANCE OF 400 ML/MIN.
PMID:821720 SCHURMAN DJ ET AL; CURR THER RES CLIN EXP 20: 194-203 (1976)
Serum half-life is 0.6 hr /From table/
Ellenhorn, M.J. and D.G. Barceloux. Medical Toxicology - Diagnosis and Treatment of Human Poisoning. New York, NY: Elsevier Science Publishing Co., Inc. 1988., p. 334
The bactericidal activity of cephapirin results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins (PBPs).
... VERY RESISTANT TO ACTION OF PENICILLINASE, FOR WHICH IT IS BOTH COMPETITIVE & NONCOMPETITIVE INHIBITOR ... IT DOES NOT SUPPRESS BREAKDOWN OF PENICILLIN G BY STAPHYLOCOCCAL PENICILLINASE. CEPHALOSPORIN C & ITS SEMISYNTHETIC CONGENERS INDUCE SYNTH OF PENICILLINASE BY B CEREUS & STAPH AUREUS. /CEPHALOSPORIN C/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1160
... ENZYME ACTING SPECIFICALLY ON CEPHALOSPORIN C TO DESTROY ITS ANTIBACTERIAL ACTIVITY. THIS SUBSTANCE, CEPHALOSPORINASE IS ALSO BETA-LACTAMASE. MOST PREPN OF ENZYME ALSO EXHIBIT PENICILLINASE ACTIVITY, & SOME MICROORGANISMS PRODUCE ONE BETA-LACTAMASE THAT ACTS ON BOTH PENICILLIN & CEPHALOSPORINS. /CEPHALOSPORIN C/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1160
Bactericidal; action depends on ability to reach and bind pencillin-binding proteins located in bacterial cytoplasmic membranes; cephalosporins inhibit bacterial septum and cell wall synthesis, probably by acylation of membrane-bound transpeptidase enzymes. This prevents cross-linkage of peptidoglycan chains, which is necessary for bacterial cell wall strength and rigidity. Also, cell division and growth are inhibited, and lysis and elongation of susceptible bacteria frequently occur. Rapidly dividing bacteria are those most susceptible to the action of cephalosporins. /Cephalosporins/
US Pharmacopeial Convention; US Pharmacopeia Dispensing Information (USP DI); Drug Information for the Health Care Professional 12th ed, VI p.846 (1992)


