



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)
0

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
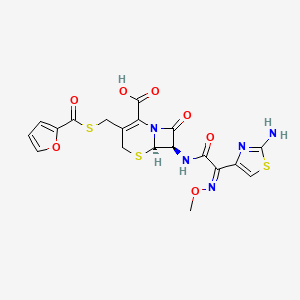

1. Ceftiofur
2. Ceftiofur Hydrochloride
3. Ceftiofur Sodium
4. U 64279a
5. U-64279e
1. Ceftiofur
2. 80370-57-6
3. Excenel
4. Ceftiofurum
5. Ceftiofur Crystalline Free Acid
6. 83jl932i1c
7. Ceftiofur (inn)
8. Ceftiofur [inn]
9. (6r,7r)-7-(2-(2-amino-4-thiazolyl)glyoxylamido)-3-(mercaptomethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7(sup 2)-(z)-(o-methyloxime), 2-furoate (ester)
10. (6r,7r)-7-[[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-(furan-2-carbonylsulfanylmethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
11. Ceftiofurum [latin]
12. Naxcel [veterinary] (tn)
13. Ceftiofur [usan:inn:ban]
14. Unii-83jl932i1c
15. Hsdb 7445
16. Ncgc00167543-01
17. (6r,7r)-7-((z)-2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-(((furan-2-carbonyl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
18. Ceftiofur [mi]
19. Ceftiofur [hsdb]
20. Ceftiofur [mart.]
21. Ceftiofur [who-dd]
22. Dsstox_cid_26702
23. Dsstox_rid_81836
24. Dsstox_gsid_46702
25. Schembl332041
26. Chembl222913
27. Dtxsid7046702
28. Hy-n7102
29. Tox21_112540
30. Bdbm50103524
31. S5356
32. Zinc38141711
33. Akos015998831
34. Ccg-269876
35. Ceftiofur [ema Epar Veterinary]
36. Db11485
37. Cas-80370-57-6
38. Ceftiofur 100 Mug/ml In Acetonitrile/water
39. Cs-0081818
40. Ceftiofur 100 Microg/ml In Acetonitrile/water
41. D07657
42. 370c576
43. A839898
44. Ceftiofur Crystalline Free Acid [green Book]
45. Brd-k82960980-003-02-7
46. (6r,7r)-7-[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetamido]-3-[(furan-2-carbonylsulfanyl)methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
47. (6r,7r)-7-[[(2z)-2-(2-amino-4-thiazolyl)-2-methoxyimino-1-oxoethyl]amino]-3-[[[2-furanyl(oxo)methyl]thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
48. (6r,7r)-7-[[(2z)-2-(2-azanyl-1,3-thiazol-4-yl)-2-methoxyimino-ethanoyl]amino]-3-(furan-2-ylcarbonylsulfanylmethyl)-8-oxidanylidene-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
49. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-3-(((2-furanylcarbonyl)thio)methyl)-8-oxo-, (6r-(6.alpha.,7.beta.(z)))-
50. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-3-(((2-furanylcarbonyl)thio)methyl)-8-oxo-, (6r-(6alpha,7beta(z)))-
51. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((2z)-(2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-3-(((2-furanylcarbonyl)thio)methyl)-8-oxo-, (6r,7r)-
| Molecular Weight | 523.6 g/mol |
|---|---|
| Molecular Formula | C19H17N5O7S3 |
| XLogP3 | 0.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 9 |
| Exact Mass | 523.02901142 g/mol |
| Monoisotopic Mass | 523.02901142 g/mol |
| Topological Polar Surface Area | 256 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 945 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
MEDICATION (VET): Ceftiofur is used in the treatment of respiratory infections in cattle and pigs.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 36: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Ceftiofur (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v36je01.htm
THERAP CAT (VET): Antibacterial
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 334
MEDICATION (VET): Ceftiofur is an injectable cephalosporin approved for respiratory disease in horses and cattle and for treatment of canine bacterial urinary tract infections caused by E coli and Proteus.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2042
MEDICATION (VET): Dogs: ... Ceftiofur sodium for injection are indicated in the treatment of urinary tract infections caused by susceptible organisms, including E. coli, P. mirabilis, and S. aureus. /Ceftiofur sodium/
Thomson/Micromedex. USP Veterinary Pharmaceutical Information Monographs: Cephalolsporins (2003). Available from, as of July 26, 2006: https://www.usp.org/audiences/veterinary/monographs/main.html
For more Therapeutic Uses (Complete) data for CEFTIOFUR (13 total), please visit the HSDB record page.
The cephalosporins are relatively nontoxic, ... . IM injections can be painful, and repeated IV administration may lead to local phlebitis. Nausea, vomiting, and diarrhea may occasionally be seen. Hypersensitivity reactions of several forms have been seen, particularly in animals with a history of acute penicillin allergy. Superinfection may arise with the use of cephalosporins, and Pseudomonas or Candida spp are likely opportunistic pathogens. /Cephalosporins/
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2065
Ceftiofur is associated with a duration and dose-related thrombocytopenia and anemia in dogs, which would not be expected with the recommended dosage regimen.
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2042
Ceftiofur concentrations in an infected and uninfected environment were compared and the efficacy of locally administered ceftiofur was evaluated in an experimental infection with Staphylococcus aureus in tissue cages. Eight ponies had tissue cages (TCs) implanted sc on each side of the neck. Into one of the cages 150 mg of ceftiofur was administered and fluid samples were taken to determine ceftiofur concentrations. After 1 week the other TC was infected with S. aureus and subsequently treated with 150 mg ceftiofur administered locally into the TC once daily for 21 days. Samples of fluid were taken to determine ceftiofur concentrations and for bacterial counts. Ceftiofur concentrations did not differ significantly in the infected and uninfected environments after single dose of 150 mg of ceftiofur. Concentrations were considerably in excess of the minimum inhibitory concentration (MIC) of the S. aureus strain used. A marked decrease of viable bacteria in tissue cage fluid (TCF) occurred. In five of seven ponies; however, the infection was not eliminated and abscess formation occurred. Therefore, local application of ceftiofur alone is not advisable for infections with S. aureus in secluded sites in horses, but should be used only with adjunctive therapy.
PMID:16420299 Bosch G et al; J Vet Pharmacol Ther 29 (1): 31-6 (2006)
Horses: Diarrhea with ceftiofur.
Thomson/Micromedex. USP Veterinary Pharmaceutical Information Monographs: Cephalolsporins (2003). Available from, as of July 26, 2006: https://www.usp.org/audiences/veterinary/monographs/main.html
Anemia and thrombocytopenia have been seen in dogs given ceftiofur at high doses (three to five times the labeled dose) or for long periods of time (5 to 6 weeks). These side effects appear to be reversible when treatment is discontinued.
Thomson/Micromedex. USP Veterinary Pharmaceutical Information Monographs: Cephalolsporins (2003). Available from, as of July 26, 2006: https://www.usp.org/audiences/veterinary/monographs/main.html
* Pigs:
- Treatment of bacterial respiratory disease associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis and Streptococcus suis.
- Treatment of septicaemia, polyarthritis or polyserositis associated with Streptococcus suis infection.
* Cattle:
- Treatment of acute interdigital necrobacillosis in cattle also known as Panaritium or foot rot.
- Treatment of acute post-partum (puerperal) metritis in cattle, in cases where treatment with another antimicrobial has failed.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
QJ01DD90
A study of 4 male and 4 female Sprague-Dawley rats treated intramuscularly with (14)C-ceftiofur (2 mg/kg bw) revealed that 55% of the administered dose was excreted in the urine and about 30% in the GI tract and feces. The major urinary metabolite was desfuroylceftiofur (DFC). The metabolism of ceftiofur was similar in calves administered (14)C-ceftiofur (2 mg/kg bw) via the i.m. route. Unmetabolized ceftiofur was also present in the urine (4.4-21% of total radioactivity).
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 36: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Ceftiofur (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v36je01.htm
A group of Sprague-Dawley rats (7/sex) received single oral doses of (14)C-ceftiofur (200 mg/kg bw) in a comparative study with calves. Approximately 55% of the total dose was recovered in the urine and the rest was present in the feces and GI tract. Plasma concentration at 6 hr was 1 mg/kg and trace amounts of ceftiofur were present in all tissues (i.e. liver, muscle and fat). The highest residue levels (0.7 mg/kg) were present in kidney.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 36: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Ceftiofur (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v36je01.htm
A study of lactating cows treated with (14)C-ceftiofur (2.3 mg/kg bw/day for 5 days) revealed that 32-38% of the radioactivity was present in the milk as free metabolites. The major metabolite was desfuroylceftiofur cysteine disulfide representing 7-9% of the total radioactivity. No parent compound was detected in the milk.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 36: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Ceftiofur (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v36je01.htm
A study of im administration of (14)C-ceftiofur in a bull revealed that 55% of the administered dose was excreted in the urine and approximately 30% in the GI tract and feces. The initial metabolite in both urine and plasma was desfuroylceftiofur. HPLC analysis of radioactive metabolites was similar to the results found in the rat studies. A number of metabolites were produced, the major metabolite (87% of total urinary metabolites) being desfuroylceftiofur acetamide conjugates. No parent compound was observed in the urine.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 36: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Ceftiofur (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v36je01.htm
For more Absorption, Distribution and Excretion (Complete) data for CEFTIOFUR (13 total), please visit the HSDB record page.
A study of 4 male and 4 female Sprague-Dawley rats treated intramuscularly with (14)C-ceftiofur (2 mg/kg bw) revealed that 55% of the administered dose was excreted in the urine and about 30% in the GI tract and faeces. The major urinary metabolite was desfuroylceftiofur (DFC). The metabolism of ceftiofur was similar in calves administered (14)C-ceftiofur (2 mg/kg bw) via the i.m. route. Unmetabolized ceftiofur was also present in the urine (4.4-21% of total radioactivity).
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 36: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Ceftiofur (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v36je01.htm
A group of Sprague-Dawley rats (7/sex) received single oral doses of (14)C-ceftiofur (200 mg/kg bw) in a comparative study with calves. Approximately 55% of the total dose was recovered in the urine and the rest was present in the feces and GI tract. ... The major urinary metabolite was ceftiofursulfoxide cysteine thioester.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 36: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Ceftiofur (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v36je01.htm
HPLC analysis of metabolites of (14)C-ceftiofur formed by arochlor-induced rat liver S-9 fractions in vitro revealed that desfuroylceftiofur was the major metabolite. Low doses (119 mg/kg bw) of ceftiofur were completely metabolized within 15 minutes. Higher doses (857 mg/kg bw) were converted to desfuroylceftiofur after 60 minutes of incubation.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 36: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Ceftiofur (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v36je01.htm
A study in 8-week old Sprague-Dawley rats (7/sex) treated with (14)C-ceftiofur (800 mg/kg bw/day) by oral gavage for 5 days revealed several urinary metabolites, including desfuroylceftiofur, ceftiofur sulfoxide, and cysteine disulfide.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 36: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Ceftiofur (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v36je01.htm
For more Metabolism/Metabolites (Complete) data for CEFTIOFUR (15 total), please visit the HSDB record page.
Six Friesian calves (3/sex) were treated with ceftiofur according to different protocols including one single im and iv injection at 1 mg/kg bw, and 5 i.m. injections at 1 mg/kg bw at 24 hr intervals. ... The half life (0.07 hr) was short due to rapid metabolism to desfuroylceftiofur. The t1/2 of desfuroylceftiofur after im and iv administration were similar (9.7 and 8.6 hr, respectively).
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 36: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Ceftiofur (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v36je01.htm
A study of 4 calves (sex and breed unspecified) administered ceftiofur intramuscularly daily for 4 days at 2 dose levels (2.2 or 4.4 mg/kg bw/day) demonstrated a plasma half life of 3.5 hr. ... Plasma half life of the metabolite desfuroylceftiofur was 9.7 h after im administration.
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 36: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Ceftiofur (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v36je01.htm
A study of 4- to 5-month old Yorkshire-Hampshire pigs (6/sex) treated with 3 daily im injections of (14)C-ceftiofur (5.2 mg/kg bw) produced similar results to those observed in rats and cattle. ... The half life of desfuroylceftiofur was 13.5 hr after im treatment and 12.2 hr after iv treatment. /Desfuroylceftiofur/
Joint FAO/WHO Expert Committee on Food Additives; WHO Food Additive Series 36: Toxicological Evaluation of Certain Veterinary Drug Residues in Food: Ceftiofur (1996). Available from, as of July 17, 2006: https://www.inchem.org/documents/jecfa/jecmono/v36je01.htm
Ceftiofur sodium is a third generation broad-spectrum cephalosporin, formulated as an intramuscular injection, which is used to treat respiratory diseases in swine, ruminants and horses. The thioester bond on ceftiofur is rapidly cleaved to give desfuroylceftiofur which is further metabolized to a disulfide dimer and various desfuroylceftiofur-protein and amino acid conjugates.
PMID:16242891 Jacobson GA; Journal of Pharmaceutical and Biomedical Analysis 40 (5): 1249-1252 (2006)
Cephalosporins ... bind to penicillin-binding proteins located beneath the cell wall and thereby interfere with the action of transpeptidase and other cell-wall enzymes. A residual antibacterial effect is also evident with the cephalosporins. /Cephalosporins/
Kahn, C.M. (Ed.); The Merck Veterinary Manual 9th ed. Merck & Co. Whitehouse Station, NJ. 2005, p. 2063


