



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
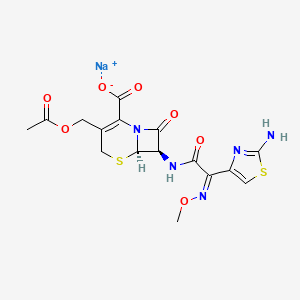

1. Benaxima
2. Biosint
3. Cefotaxim
4. Cefotaxime
5. Cefradil
6. Cephotaxim
7. Claforan
8. Fotexina
9. Hr 756
10. Hr-756
11. Hr756
12. Kendrick
13. Klaforan
14. Primafen
15. Ru 24756
16. Ru-24756
17. Ru24756
18. Sodium, Cefotaxime
19. Taporin
1. 64485-93-4
2. Cefotaxime Sodium Salt
3. Sodium Cefotaxime
4. Hr 756
5. Cefotaxime
6. Ru 24756
7. (+)-cefotaxime Sodium Salt
8. Hr-756
9. Cefotaxime (sodium)
10. Ru-24756
11. Mls000028559
12. Chebi:3498
13. 258j72s7tz
14. Sodium 7-(2-(2-amino-4-thiazolyl)-2-methoxyiminoacetamido)cephalosporanate
15. Cefotax
16. Nsc-756666
17. Sodium;(6r,7r)-3-(acetyloxymethyl)-7-[[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
18. Smr000058812
19. Tolycar
20. Pretor
21. Sodium (6r,7r)-7-(2-(2-amino-4-thiazolyl)glyoxylamido)-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylate 7(sup 2)-(z)-(o-methyloxime), Acetate (ester)
22. Sodium;(6r,7r)-3-(acetyloxymethyl)-7-[[(2e)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
23. Cefotaxime (sodium Salt)
24. Ralopar
25. Tolycor
26. Zariviz
27. Claforan Sodium
28. Mls002222330
29. Cefotaxime Na-salt
30. Cefotaxime Sodium Salt (syn-isomer)
31. Smr001307269
32. Mfcd00079073
33. Unii-258j72s7tz
34. Cefotaxim Sodium
35. Claforan (tn)
36. Prestwick_823
37. Cefotaxime Sodium [usan:usp:jan]
38. Einecs 264-915-9
39. Cefotaxime, Sodium Salt
40. Opera_id_1945
41. Chembl1010
42. Schembl41092
43. Cefotaxime And Dextrose 2.4% In Plastic Container
44. Cefotaxime And Dextrose 3.9% In Plastic Container
45. Mls001077352
46. Mls001333684
47. Sym-methoxyimino Cephalosporin
48. Claforan In Dextrose 5% In Plastic Container
49. Cefotaxime Sodium [jan]
50. Cefotaxime Sodium (jp17/usp)
51. Cefotaxime Sodium [usan]
52. Dtxsid4046991
53. Cefotaxime Sodium [vandf]
54. Cefotaxime Sodium [mart.]
55. Claforan In Sodium Chloride 0.9% In Plastic Container
56. Hms1568k20
57. Hms2095k20
58. Hms2233j21
59. Hms3260h18
60. Hms3712k20
61. Cefotaxime Sodium [usp-rs]
62. Cefotaxime Sodium [who-dd]
63. Amy40166
64. Cefotaxime Sodium Salt [mi]
65. Hy-a0088
66. Tox21_500278
67. S4517
68. Akos015951272
69. Ccg-220139
70. Ccg-221582
71. Cefotaxime Sodium [ep Impurity]
72. Cefotaxime Sodium [orange Book]
73. Cs-4297
74. Ks-1416
75. Nsc 756666
76. Cefotaxime Sodium [usp Impurity]
77. Cefotaxime Sodium [usp Monograph]
78. Ncgc00093734-01
79. Ncgc00093734-02
80. Ncgc00260963-01
81. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 3-((acetyloxy)methyl)-7-(((2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-8-oxo-, Monosodium Salt, (6r-(6alpha,7beta(z)))-
82. Smr000653480
83. Eu-0100278
84. A16005
85. C 7912
86. C08113
87. D00919
88. Cefadroxil, Antibiotic For Culture Media Use Only
89. Q-200807
90. Cefotaxime Sodium Salt, Potency: 916-964 Mug Per Mg
91. Cefotaxime Sodium, European Pharmacopoeia (ep) Reference Standard
92. Cefotaxime Sodium Salt, Plant Cell Culture Tested, Bioreagent, Powder
93. Cefotaxime Sodium, United States Pharmacopeia (usp) Reference Standard
94. Cefotaxime For Peak Identification, European Pharmacopoeia (ep) Reference Standard
95. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 3-((acetyloxy)methyl)-7-(((2-amino-4-thiazolyl)(methoxyimino)acetyl)amino)-8-oxo-, Monosodium Salt, (6r-(6.alpha.,7.beta.(z)))-
96. Sodium (6r,7r)-3-(acetoxymethyl)-7-((e)-2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
97. Sodium (6r,7r)-3-(acetoxymethyl)-7-{[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate
98. Sodium (6r-(6alpha,7beta(z)))-3-(acetoxymethyl)-7-((2-aminothiazol-4-yl)(methoxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylate
99. Sodium 3-(acetoxymethyl)-7beta-{[(2z)-2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3,4-didehydrocepham-4-carboxylate
| Molecular Weight | 477.5 g/mol |
|---|---|
| Molecular Formula | C16H16N5NaO7S2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 8 |
| Exact Mass | 477.03888450 g/mol |
| Monoisotopic Mass | 477.03888450 g/mol |
| Topological Polar Surface Area | 230 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 839 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Cefotaxime sodium |
| Drug Label | Sterile CLAFORAN (cefotaxime sodium) is a semisynthetic, broad spectrum cephalosporin antibiotic for parenteral administration. It is the sodium salt of 7-[2-(2-amino-4-thiazolyl) glyoxylamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo [4.2.0] oct-... |
| Active Ingredient | Cefotaxime sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 500mg base/vial; eq 10gm base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Wockhardt; Hospira; Lupin |
| 2 of 4 | |
|---|---|
| Drug Name | Claforan |
| Drug Label | Sterile CLAFORAN (cefotaxime sodium) is a semisynthetic, broad spectrum cephalosporin antibiotic for parenteral administration. It is the sodium salt of 7-[2-(2-amino-4-thiazolyl) glyoxylamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo [4.2.0] oct-... |
| Active Ingredient | Cefotaxime sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 500mg base/vial; eq 10gm base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 3 of 4 | |
|---|---|
| Drug Name | Cefotaxime sodium |
| Drug Label | Sterile CLAFORAN (cefotaxime sodium) is a semisynthetic, broad spectrum cephalosporin antibiotic for parenteral administration. It is the sodium salt of 7-[2-(2-amino-4-thiazolyl) glyoxylamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo [4.2.0] oct-... |
| Active Ingredient | Cefotaxime sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 500mg base/vial; eq 10gm base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Wockhardt; Hospira; Lupin |
| 4 of 4 | |
|---|---|
| Drug Name | Claforan |
| Drug Label | Sterile CLAFORAN (cefotaxime sodium) is a semisynthetic, broad spectrum cephalosporin antibiotic for parenteral administration. It is the sodium salt of 7-[2-(2-amino-4-thiazolyl) glyoxylamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo [4.2.0] oct-... |
| Active Ingredient | Cefotaxime sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2gm base/vial; eq 500mg base/vial; eq 10gm base/vial; eq 1gm base/vial |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DD - Third-generation cephalosporins
J01DD01 - Cefotaxime




