



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
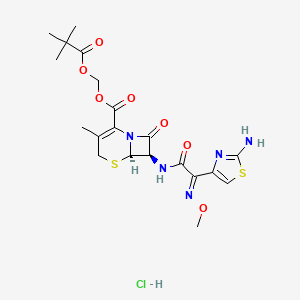

1. Cefetamet Pivoxil Hydrochloride
2. 111696-23-2
3. 2,2-dimethylpropanoyloxymethyl (6r,7r)-7-[[(2e)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate;hydrochloride
4. Ncgc00181788-01
5. Dsstox_cid_28516
6. Dsstox_rid_82788
7. Dsstox_gsid_48590
8. Chembl2355376
9. Dtxsid1048590
10. Tox21_112919
11. Akos032949665
12. Cas-111696-23-2
13. Q27114287
14. Toluene-4-sulfonicacid(s)-1-chlorocarbonyl-ethylester
15. (pivaloyloxy)methyl (6r,7r)-7-((z)-2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate Hydrochloride
16. Cefetamet Pivoxil Hydrochloride; (6r,7r)-7-[[(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid (2,2-dimethyl-1-oxopropoxy)methyl Ester Hydrochloride
| Molecular Weight | 548.0 g/mol |
|---|---|
| Molecular Formula | C20H26ClN5O7S2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 10 |
| Exact Mass | 547.0962182 g/mol |
| Monoisotopic Mass | 547.0962182 g/mol |
| Topological Polar Surface Area | 216 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 933 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |


