


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
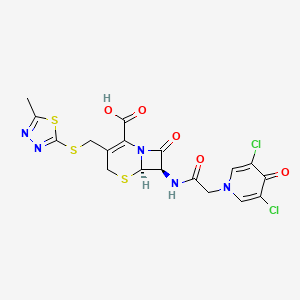

1. 7-(2-(3,5-dichloro-4-oxo-1- Pyridyl)acetamido)-3-(5-methyl-1,3,4-thiadiazol- 2-ylthiomethyl)-3-cephem-4-carboxylic Acid
2. Cefazedone Sodium
3. Cefazedone, Monosodium Salt
4. Emd 30 087
5. Emd30087
6. Refosporen
7. Refosporin
1. 56187-47-4
2. Refosporen
3. Refosporene
4. Cefazedona
5. Cefazedonum
6. (6r,7r)-7-[[2-(3,5-dichloro-4-oxopyridin-1-yl)acetyl]amino]-3-[(5-methyl-1,3,4-thiadiazol-2-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
7. Cefazedone (inn)
8. Emd-30087
9. 7y86x0d799
10. (6r,7r)-7-(2-(3,5-dichloro-4-oxo-1(4h)-pyridyl)acetamido)-3-(((5-methyl-1,3,4-thiadiazol-2-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)-oct-2-ene-2-carboxylic Acid
11. Cefazedone [inn]
12. Cefazedone [inn:ban]
13. Cefazedonum [inn-latin]
14. Cefazedona [inn-spanish]
15. (6r,7r)-7-(2-(3,5-dichloro-4-oxopyridin-1(4h)-yl)acetamido)-3-(((5-methyl-1,3,4-thiadiazol-2-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
16. Refosporin Sodium Salt
17. Cefazedone, Sodium Salt
18. Brn 1097746
19. Cefazedon
20. Cefazedone Acid
21. Unii-7y86x0d799
22. Refosporen;cefazedonum
23. Nsc304118
24. Cefazedone [mi]
25. Cefazedone (*sodium Salt*)
26. Cefazedone [who-dd]
27. Refosporin (*sodium Salt*)
28. Schembl49523
29. Chembl2107636
30. Gtpl12203
31. Dtxsid50204733
32. Chebi:131731
33. Zinc1567521
34. S4874
35. Ccg-269995
36. Db13778
37. (6r,7r)-7-[[2-(3,5-dichloro-4-oxo-1-pyridyl)acetyl]amino]-3-[(5-methyl-1,3,4-thiadiazol-2-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
38. (6r-trans)7-(((3,5-dichloro-4-oxo-1(4h)-pyridinyl)acetyl)amino)-3-(((5-methyl-1,3,4-thiadiazol-2-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oxy-2-ene-2-carbox Ylic Acid
39. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-(((3,5-dichloro-4-oxo-1(4h)-pyridinyl)acetyl)amino)-3-(((5-methyl-1,3,4-thiadiazol-2-yl)thio)methyl)-8-oxo-, (6r-trans)-
40. As-75724
41. Hy-121144
42. Cs-0079538
43. D07237
44. D95160
45. 187c474
46. A936727
47. Q5057222
48. (6r,7r)-7-(2-(3,5-dichloro-4-oxopyridin-1(4h)-yl)acetamido)-3-(((5-methyl-1,3,4-thiadiazol-2-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylicacid
49. (6r,7r)-7-[2-(3,5-dichloro-4-oxopyridin-1(4h)-yl)acetamido]-3-{[(5-methyl-1,3,4-thiadiazol-2-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid
50. 5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic Acid, 7-((2-(3,5-dichloro-4-oxo-1(4h)-pyridinyl)acetyl)amino)-3-(((5-methyl-1,3,4-thiadiazol-2-yl)thio)methyl)-8-oxo-, (6r,7r)-
51. 5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid, 7-[[(3,5-dichloro-4-oxo-1(4h)-pyridinyl)acetyl]amino]-3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio]methyl]-8-oxo-, Monosodium Salt, (6r-trans)-
52. 7-{[3,5-dichloro-4-oxopyridin-1(4h)-yl]acetamido}-3-{[(5-methyl-1,3,4-thiadiazol-2-yl)sulfanyl]methyl}-3,4-didehydrocepham-4-carboxylic Acid
| Molecular Weight | 548.4 g/mol |
|---|---|
| Molecular Formula | C18H15Cl2N5O5S3 |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 7 |
| Exact Mass | 546.9612375 g/mol |
| Monoisotopic Mass | 546.9612375 g/mol |
| Topological Polar Surface Area | 212 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 984 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DB - First-generation cephalosporins
J01DB06 - Cefazedone


