



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0

1. Aquacel
2. Aquaplast
3. Carboxymethyl Cellulose
4. Carboxymethylcellulose
5. Carboxymethylcellulose Sodium
6. Carboxymethylcellulose, Sodium
7. Carmellose Sodium
8. Cellolax
9. Cellulose, Carboxymethyl
10. Cethylose
11. Croscarmellose Sodium
12. Polycell
13. Ruspol
14. Sodium Carboxymethylcellulose
15. Sodium, Carboxymethylcellulose
16. Sodium, Carmellose
17. Sodium, Croscarmellose
1. Carboxymethylcellulose
2. Carboxymethyl Cellulose
3. 9000-11-7
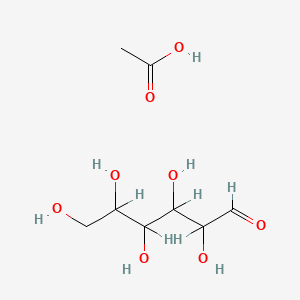
4. Acetic Acid;2,3,4,5,6-pentahydroxyhexanal
5. Cellulose, Carboxymethyl Ether
6. Cellulose Cm
7. Colloresine
8. Carmellose
9. Almelose
10. Apergel
11. Carbose
12. Duodcel
13. Thylose
14. Apeyel
15. Carboxymethylcellulose Cellulose Carboxymethyl Ether
16. Cm-cellulose
17. Glycocel Ta
18. Kmts
19. 7h (carbohydrate)
20. Cellulose Gum 7h
21. Celluloseglycolic Acid
22. Carboximethylcellulosum
23. Carboxymethylcellulosum
24. Croscarmellosum [latin]
25. Cellulose Carboxymethylate
26. Carmellosum [inn-latin]
27. Carmelosa [inn-spanish]
28. Cmc-4lf
29. Cellulose, (carboxymethyl)
30. Carboxymethyl Cellulose Ether
31. Carmellosum
32. Croscarmellosum
33. Croscarmellosum [inn-latin]
34. Croscarmelosa
35. Croscarmelosa [inn-spanish]
36. Glycolic Acid Cellulose Ether
37. Carmelosa
38. Fema No. 2239
39. Carboxymethylated Cellulose Pulp
40. Carmellose [inn]
41. Cellulose, Ether With Glycolic Acid
42. Intrasite Gel (tn)
43. .carboxymethylcellulose
44. Acetic Acid, Hydroxy-, Cellulose Ether
45. Croscarmellose (inn)
46. Unii-05jzi7b19x
47. 7h
48. Schembl177710
49. Carmellose (jp17/nf/inn)
50. 05jzi7b19x
51. Ft-0623543
52. D07622
53. Q411030
54. 191616-54-3
55. 196886-89-2
| Molecular Weight | 240.21 g/mol |
|---|---|
| Molecular Formula | C8H16O8 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 240.08451746 g/mol |
| Monoisotopic Mass | 240.08451746 g/mol |
| Topological Polar Surface Area | 156 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 169 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 4 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Indicated for the symptomatic relief of burning, irritation and discomfort of the eyes due to dryness or exposure to wind or sun.
In a randomized clinical study of patients with mild or moderate forms of eye dryness, ophthalmic treatment with sodium carboxymethylcellulose resulted in a diminished frequency of symptoms compared to the placebo group. Carboxymethylcellulose interacts with human corneal epithelial cells to facilitate corneal epithelial wound healing and attenuate eye irritation in a dose-dependent manner. It exhibits protective actions on the ocular surface in various applications; it mediates cytoprotective effects on the ocular surface when applied prior to contact lenses and reduces the incidence of epithelial defects during LASIK.
Laxatives
Agents that produce a soft formed stool, and relax and loosen the bowels, typically used over a protracted period, to relieve CONSTIPATION. (See all compounds classified as Laxatives.)
Absorption
No pharmacokinetic data available.
Route of Elimination
No pharmacokinetic data available.
Volume of Distribution
No pharmacokinetic data available.
Clearance
No pharmacokinetic data available.
No pharmacokinetic data available.
No pharmacokinetic data available.
Carboxymethylcellulose binds to the surface of corneal epithelial cells via its glucopyranose subunits binding to glucose receptors GLUT-1. The residence time of carboxymethylcellulose bound to corneal cells is approximately 2 hours as indicated by a short-term binding assay. Binding of carboxymethylcellulose to the matrix proteins stimulated corneal epithelial cell attachment, migration, and re-epithelialization of corneal wounds.


