



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
0
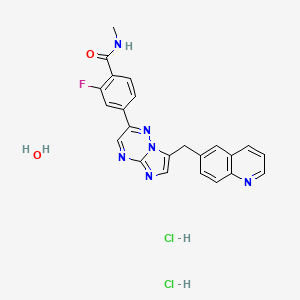

1. 2-fluoro-4-(7-((quinolin-6-yl)methyl)imidazo(1,2-b)(1,2,4)triazin-2-yl)benzoic Acid
2. 2-fluoro-4-(7-((quinolin-6-yl)methyl)imidazo(1,2-b)-(1,2,4)triazin-2-yl)benzamide
3. 2-fluoro-n-methyl-4-(7-((quinolin-6-yl)methyl)imidazo(1,2- B)(1,2,4)triazin-2-yl)benzamide Dihydrochloride Monohydrate
4. 2-fluoro-n-methyl-4-(7-(quinolin-6-yl-methyl)imidazo(1,2-b)(1,2,4)triazin-2-yl)benzamide
5. 2-fluoro-n-methyl-4-(7-(quinolin-6-ylmethyl)imidazo(1,2-b)(1,2,4)triazin-2-yl)benzamide
6. 2-fluoro-n-methyl-4-(7-(quinolin-6-ylmethyl)imidazo(1,2-b)(1,2,4)triazin-2-yl)benzamide Dihydrochloride
7. Capmatinib
8. Capmatinib Dihydrochloride
9. Capmatinib Dihydrochloride Monohydrate
10. Capmatinib Hydrochloride Anhydrous
11. Capmatinib Metabolite M13
12. Capmatinib Metabolite M18
13. Cmc-583
14. Cmc583
15. Cnj-294
16. Cnj294
17. Inc-280
18. Inc280
19. Incb-28060
20. Incb-28060 Free Base
21. Incb28060
22. Nvp-inc280
23. Nvp-inc280-nx
24. Tabrecta
1. 1865733-40-9
2. Nvp-inc280-aaa
3. Capmatinib Hydrochloride [usan]
4. C2a374o70x
5. Capmatinib (dihydrochloride Hydrate)
6. Tabrecta
7. Capmatinib Dihydrochloride Monohydrate
8. Capmatinib Hydrochloride (usan)
9. Capmatinib Hydrochloride Hydrate (jan)
10. 2-fluoro-n-methyl-4-(7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl)benzamide Dihydrochloride Hydrate
11. Capmatinib Hydrochloride Hydrate [jan]
12. Tabrecta (tn)
13. Capmatinib 2hcl.h2o
14. Capmatinib 2hcl Hydrate
15. Inc-280 Hydrochloride
16. Incb28060 Hydrochloride
17. Unii-c2a374o70x
18. Capmatinib Hydrochloride Hydrate
19. Chembl3989937
20. Dtxsid401027869
21. Hy-13404c
22. Capmatinib Hydrochloride [who-dd]
23. Capmatinib Hydrochloride Monohydrate
24. Cs-0198834
25. Capmatinib Hydrochloride [orange Book]
26. D10891
27. Q27895918
28. 2-fluoro-n-methyl-4-(7-((quinolin-6-yl)methyl)imidazo(1,2- B)(1,2,4)triazin-2-yl)benzamide Dihydrochloride Monohydrate
29. Benzamide, 2-fluoro-n-methyl-4-(7-(6-quinolinylmethyl)imidazo(1,2-b)(1,2,4)triazin-2-yl)-, Hydrochloride, Hydrate (1:2:1)
| Molecular Weight | 503.4 g/mol |
|---|---|
| Molecular Formula | C23H21Cl2FN6O2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 502.1087075 g/mol |
| Monoisotopic Mass | 502.1087075 g/mol |
| Topological Polar Surface Area | 86.1 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 637 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
Tabrecta as monotherapy is indicated for the treatment of adult patients with advanced non small cell lung cancer (NSCLC) harbouring alterations leading to mesenchymal epithelial transition factor gene exon 14 (METex14) skipping, who require systemic therapy following prior treatment with immunotherapy and/or platinum based chemotherapy.
L01EX17


