


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
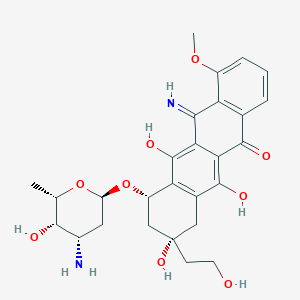

1. 5-imino-13-deoxydoxorubicin
2. Gpx-150
1. Vi79rd8vnn
2. 236095-26-4
3. Gpx-150
4. Gpx150
5. Unii-vi79rd8vnn
6. Camsirubicin [inn]
7. Camsirubicin [who-dd]
8. 5-imino-13-deoxydoxorubicin
9. (8r,10s)-10-((3-amino-2,3,6-trideoxy-.alpha.-l-lyxo-hexopyranosyl)oxy)-7,9,10,12-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyethyl)-12-imino-1-methoxy-5(8h)-naphthacenone
10. (8r,10s)-10-((3-amino-2,3,6-trideoxy-.alpha.-l-lyxohexopyranosyl) Oxy)-6,8,11-trihydroxy-8-(2-hydroxyethyl)- 12-imino-1-methoxy-7,9,10,12-tetrahydrotetracen-5(8h)- One
11. 5(8h)-naphthacenone, 10-((3-amino-2,3,6-trideoxy-.alpha.-l-lyxo-hexopyranosyl)oxy)-7,9,10,12-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyethyl)-12-imino-1-methoxy-, (8r,10s)-
12. 5(8h)-naphthacenone, 10-((3-amino-2,3,6-trideoxy-alpha-l-lyxo-hexopyranosyl)oxy)-7,9,10,12-tetrahydro-6,8,11-trihydroxy-8-(2-hydroxyethyl)-12-imino-1-methoxy-, (8r,10s)-
| Molecular Weight | 528.5 g/mol |
|---|---|
| Molecular Formula | C27H32N2O9 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 5 |
| Exact Mass | 528.21078060 g/mol |
| Monoisotopic Mass | 528.21078060 g/mol |
| Topological Polar Surface Area | 196 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 906 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


