



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
JP
0
Other Listed Suppliers
0
0
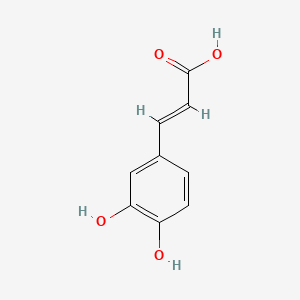

1. 3,4-dihydroxycinnamic Acid
2. Caffeic Acid, (e)-isomer
3. Caffeic Acid, (z)-isomer
4. Caffeic Acid, Monosodium Salt
5. Cis-caffeic Acid
6. Sodium Caffeate
7. Trans-caffeic Acid
1. 3,4-dihydroxycinnamic Acid
2. 331-39-5
3. Trans-caffeic Acid
4. 501-16-6
5. 3,4-dihydroxybenzeneacrylic Acid
6. 3-(3,4-dihydroxyphenyl)acrylic Acid
7. (e)-3-(3,4-dihydroxyphenyl)acrylic Acid
8. Cinnamic Acid, 3,4-dihydroxy-
9. (2e)-3-(3,4-dihydroxyphenyl)prop-2-enoic Acid
10. 3-(3,4-dihydroxyphenyl)-2-propenoic Acid
11. (e)-3,4-dihydroxycinnamic Acid
12. 2-propenoic Acid, 3-(3,4-dihydroxyphenyl)-
13. Trans-caffeate
14. (e)-3-(3,4-dihydroxyphenyl)prop-2-enoic Acid
15. 4-(2-carboxyethenyl)-1,2-dihydroxybenzene
16. 3,4-dihydroxy-trans-cinnamate
17. Caffeicacid
18. 3-(3,4-dihydroxyphenyl)propenoic Acid
19. 4-(2'-carboxyvinyl)-1,2-dihydroxybenzene
20. Nsc 57197
21. Caffeate
22. 2-propenoic Acid, 3-(3,4-dihydroxyphenyl)-, (e)-
23. 3-(3,4-dihydroxy Phenyl)-2-propenoic Acid
24. Nsc-57197
25. Chembl145
26. Nsc-623438
27. U2s3a33kvm
28. Ai3-63211
29. Mls000069738
30. 71693-97-5
31. Chebi:16433
32. Trans-3,4-dihydroxycinnamic Acid
33. Nsc57197
34. 331-89-5
35. Smr000058214
36. (2e)-3-(3,4-dihydroxyphenyl)acrylic Acid
37. 2-propenoic Acid, 3-(3,4-dihydroxyphenyl)-, (2e)-
38. Mfcd00004392
39. Caffeic Acid - Natural
40. Ccris 847
41. Hsdb 7088
42. Sr-01000000203
43. Einecs 206-361-2
44. Unii-u2s3a33kvm
45. Caffeic Acid Dehydrogenation Homopolymer
46. Chebi:36281
47. Caffeic-acid
48. Caffeic Acid Pure
49. Caffeic Acid, 1
50. Caffeic Acid 1000 Microg/ml In Acetone
51. 3,4-dihydroxycinnamic Acid (caffeic Acid)
52. Caffeic Acid Polymer
53. Caffeic Acid,(s)
54. Caffeic Acid, Trans-
55. 3,4-dihydroxycinnamate
56. Caffeic Acid Natural
57. Opera_id_1700
58. Caffeic Acid [mi]
59. Caffeic Acid [dsc]
60. Cinnamic Acid,4-dihydroxy-
61. 3,4-dihydroxycinnamic Acid, Predominantly Trans
62. Caffeic Acid [hsdb]
63. Caffeic Acid [iarc]
64. Caffeic Acid [inci]
65. 3,4-dihydroxybenzeneacrylate
66. Schembl23358
67. Mls001076493
68. Mls002207132
69. Mls002222302
70. Mls006011849
71. Bidd:er0456
72. Spectrum1503987
73. Caffeic Acid [who-dd]
74. 2-propenoic Acid,3-(3,4-dihydroxyphenyl)-, (2e)-
75. 3,4-dihydroxycinnamate, Xvii
76. Bdbm4375
77. Cid_689043
78. Gtpl5155
79. 3-(3,4-dihydroxyphenyl)-2-propenoic Acid, Homopolymer
80. Zinc58172
81. 2-propenoic Acid, 3-(3,4-dihydroxyphenyl)-, Homopolymer
82. Amy3943
83. Dtxsid901316055
84. Hms2235g09
85. Hms3260j17
86. Hms3649o17
87. Bcp28271
88. Hy-n0172
89. Tox21_500208
90. Bbl012113
91. Caffeic Acid - Cas 331-39-5
92. Ccg-38895
93. Nsc623438
94. S2277
95. Stk397812
96. Caffeic Acid, >=98.0% (hplc)
97. 2-propenoic Acid,4-dihydroxyphenyl)-
98. Akos000144463
99. Ac-8006
100. Cs-8205
101. Db01880
102. Lp00208
103. Sdccgmls-0002982.p003
104. Sdccgsbi-0050196.p004
105. Ncgc00017364-04
106. Ncgc00017364-05
107. Ncgc00017364-06
108. Ncgc00017364-07
109. Ncgc00017364-08
110. Ncgc00017364-09
111. Ncgc00017364-10
112. Ncgc00017364-11
113. Ncgc00017364-12
114. Ncgc00017364-13
115. Ncgc00017364-22
116. Ncgc00022654-03
117. Ncgc00022654-04
118. Ncgc00022654-05
119. Ncgc00022654-06
120. Ncgc00022654-07
121. Ncgc00022654-08
122. Ncgc00022654-09
123. Ncgc00260893-01
124. (e)-3-(3,4-dihydroxyphenyl)acrylicacid
125. As-10895
126. Bp-30112
127. Smr004703501
128. Xc164210
129. Caffeic Acid, Purum, >=95.0% (hplc)
130. Ab00490047
131. Eu-0100208
132. N1735
133. Sw197202-3
134. 2-morpholin-4-yl-isonicotinicacidhydrochloride
135. C 0625
136. C-1500
137. C01197
138. C01481
139. (2e)-3-(3,4-dihydroxyphenyl)-2-propenoic Acid
140. 3-(3,4-dihydroxyphenyl)-2-propenoic Acid Polymer
141. 331c395
142. A851723
143. Q414116
144. Sr-01000000203-2
145. Sr-01000000203-6
146. Sr-01000000203-7
147. Sr-01000000203-8
148. Brd-k09900591-001-06-9
149. Sr-01000000203-13
150. Caffeic Acid (constituent Of Black Cohosh) [dsc]
151. F3096-1708
152. 8b3e4da7-f3b0-4972-a315-2e387071737f
153. Trans-caffeic Acid, Certified Reference Material, Tracecert(r)
154. Caffeic Acid, Matrix Substance For Maldi-ms, >=99.0% (hplc)
155. Caffeic Acid, United States Pharmacopeia (usp) Reference Standard
156. Caffeic Acid, Matrix Substance For Maldi-ms, >=99.0% (hplc), Powder, Light Beige
| Molecular Weight | 180.16 g/mol |
|---|---|
| Molecular Formula | C9H8O4 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 180.04225873 g/mol |
| Monoisotopic Mass | 180.04225873 g/mol |
| Topological Polar Surface Area | 77.8 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 212 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Enzymes involved in its /caffeic acid/ metabolism have not been identified. In the following, caffeic (CA), chlorogenic (CGA), and dihydrocaffeic (DHCA) acids were incubated with hepatocytes and shown to undergo metabolism by cytochrome P450, catechol-O-methyltransferase (COMT), and beta-oxidation enzymes. Ferulic (FA) or dihydroferulic (DHFA) acids, formed as the result of CA- or DHCA-O-methylation by COMT, were also O-demethylated by CYP1A1/2 but not CYP2E1. DHCA or DHFA also underwent side chain dehydrogenation to form CA and FA, respectively, which was prevented by thioglycolic acid, an inhibitor of the beta-oxidation enzyme acyl CoA dehydrogenase. The rates of glutathione conjugate formation catalyzed by NADPH/microsomes (CYP2E1) in decreasing order DHCA>CA>CGA trend which was in reverse order to the rates of their O-methylation by COMT. The CA- and DHCA-o-quinones formed by NADPH/P450 likely inhibited COMT but can readily form glutathione conjugates. CA, DHCA and DHFA were inter-metabolized to each other and to FA by isolated rat hepatocytes whereas FA was metabolized only to CA but not to DHCA or DHFA. CA, DHCA, FA, DHFA and CGA showed a dose-dependent hepatocyte toxicity and the LD(50) (2 h), determined were in decreasing order of effectiveness DHCA>CA>DHFA>CGA>FA. In summary, evidence has been provided that O-methylation, GSH conjugation, hydrogenation and dehydrogenation are involved in the hepatic metabolism of CA and DHCA. The O-methylation pathway for CA and DHCA is a detoxification route whereas o-quinones formation catalyzed by P450 is the toxification route.
Moridani MY et al; Toxicol Letters 133(2-3): 141-151 (2002)
In rats, chlorogenic acid is hydrolysed in the stomach and intestine to caffeic and quinic acids. A number of metabolites have been identified. Glucuronides of meta-coumaric acid and meta-hydroxyhippuric acid appear to be the main metabolites in humans. After oral administration of caffeic acid to human volunteers, O-methylated derivatives (ferulic, dihydroferulic and vanillic acids) were excreted rapidly in the urine, while the meta-hydroxyphenyl derivatives appeared later. The dehydroxylation reactions were ascribed to the action of intestinal bacteria.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V56 125 (1993)
Caffeic Acid has known human metabolites that include (2S,3S,4S,5R)-6-[4-[(E)-2-carboxyethenyl]-2-hydroxyphenoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid and (2S,3S,4S,5R)-6-[5-[(E)-2-carboxyethenyl]-2-hydroxyphenoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Caffeic acid phenethyl ester (CAPE) was synthesized from caffeic acid and phenethyl alcohol (ratio 1:5) at room temperature with dicyclohexyl carbodiimide (DCC) as a condensing reagent. The yield was about 38%. CAPE was found to arrest the growth of human leukemia HL-60 cells. It also inhibits DNA, RNA and protein synthesis in HL-60 cells with IC50 of 1.0 M, 5.0 M and 1.5 M, respectively.
PMID:8973597 Jain-Hong C et al; Cancer Letters 108(2): 211-214 (1996)
In an attempt to understand the antihyperglycemic action of caffeic acid, the myoblast C2C12 cells were employed to investigate the glucose uptake in the present study. Caffeic acid enhanced the uptake of radioactive glucose into C2C12 cells in a concentration-dependent manner. Similar effect of phenylephrine on the uptake of radioactive glucose was also observed in C2C12 cells. Prazosin attenuated the action of caffeic acid in a way parallel to the blockade of phenylephrine. Effect of caffeic acid on alpha1-adrenoceptors was further supported by the displacement of [3H]prazosin binding in C2C12 cells. Moreover, the glucose uptake-increasing action of phenylephrine in C2C12 cells was inhibited by the antagonists of alpha1A-adrenoceptors, both tamsulosin and WB 4101, but not by the antagonist of alpha1B-adrenoceptors, chlorethylclonidine (CEC). The presence of alpha1A-adrenoceptors in C2C12 cells can thus be considered. Similar inhibition of the action of caffeic acid was also obtained in C2C12 cells co-incubating these antagonists. An activation of alpha1A-adrenoceptors seems responsible for the action of caffeic acid in C2C12 cells. In the presence of U73312, the specific inhibitor of phospholipase C, caffeic acid-stimulated uptake of radioactive glucose into C2C12 cells was reduced in a concentration-dependent manner and it was not affected by U73343, the negative control of U73312. Moreover, chelerythrine and GF 109203X diminished the action of caffeic acid at concentrations sufficient to inhibit protein kinase C. Therefore, the obtained data suggest that an activation of alpha1A-adrenoceptors in C2C12 cells by caffeic acid may increase the glucose uptake via phospholipase C-protein kinase C pathway.
PMID:10961374 Cheng J et al; Naunyn Schmiedebergs Arch Pharmacol 362(2): 122-127 (2000)
Caffeic acid (CA, 3,4-dihydroxycinnamic acid), at 2% in the diet, had been shown to be carcinogenic in forestomach and kidney of F344 rats and B6C3F1 mice. Based on its occurrence in coffee and numerous foods and using a linear interpolation for cancer incidence between dose 0 and 2%, the cancer risk in humans would be considerable. In both target organs, tumor formation was preceded by hyperplasia, which could represent the main mechanism of carcinogenic action. The dose-response relationship for this effect was investigated in male F344 rats after 4-week feeding with CA at different dietary concentrations (0, 0.05, 0.14, 0.40, and 1.64%). Cells in S-phase of DNA replication were visualized by immunohistochemical analysis of incorporated 5-bromo-2'-deoxyuridine (BrdU), 2 hr after intraperitoneal injection. In the forestomach, both the total number of epithelial cells per millimeter section length and the unit length labeling index of BrdU-positive cells (ULLI) were increased, about 2.5-fold, at 0.40 and 1.64%. The lowest concentration (0.05%) had no effect. At 0.14%, both variables were decreased by about one-third. In the kidney, the labeling index in proximal tubular cells also indicated a J-shaped (or U-shaped) dose response with a 1.8-fold increase at 1.64%. In the glandular stomach and in the liver, which are not target organs, no dose-related effect was seen. The data show a good correlation between the organ specificity for cancer induction and stimulation of cell division. With respect to the dose-response relationship and the corresponding extrapolation of the animal tumor data to a human cancer risk, a linear extrapolation appears not to be appropriate.
Lutz U et al; Fundamental Applied Toxicology 39(2): 131-137 (1997)


