



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0

1. Allylbarbital
2. Butalbital, Monosodium Salt
1. Allylbarbital
2. Itobarbital
3. Tetrallobarbital
4. Alisobumal
5. Allylbarbitone
6. Butalbarbital
7. Allylbarbituric Acid
8. 77-26-9
9. Sandoptal
10. Profundal
11. Allylisobutylbarbital
12. Butalbitalum
13. 5-allyl-5-isobutylbarbituric Acid
14. Iso-butylallylbarbituric Acid
15. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-(2-methylpropyl)-5-(2-propenyl)-
16. 5-allyl-5-(2'-methyl-n-propyl) Barbituric Acid
17. Butalbital Ciii
18. 5-allyl-5-(2-methylpropyl)barbituric Acid
19. 5-(2-methylpropyl)-5-prop-2-enyl-1,3-diazinane-2,4,6-trione
20. 5-allyl-5-isobutyl-2,4,6(1h,3h,5h)-pyrimidinetrione
21. Khs0az4jvk
22. Barbituric Acid, 5-allyl-5-isobutyl-
23. Alisobumalum
24. 5-allyl-5-isobutyl-pyrimidine-2,4,6-trione
25. Chebi:102524
26. 5-isobutyl-5-allylbarbituric Acid
27. Butalbitale
28. Butalbitale [dcit]
29. Allylisobutylbarbiturate
30. Butalbitalum [inn-latin]
31. Allylisobutylbarbituric Acid
32. Isobutylallylbarbituric Acid
33. Sandoptal (tn)
34. Butalbital (usp/inn)
35. Unii-khs0az4jvk
36. Isobutylallylbarturic Acid
37. Einecs 201-017-8
38. Butalbital [usan:usp:inn]
39. Hsdb 7853
40. Butalbital, Usp
41. Axocet (salt/mix)
42. Butalbital [mi]
43. Butalbital [inn]
44. Butalbital [hsdb]
45. Butalbital [usan]
46. Butalbital [vandf]
47. Chembl454
48. Butalbital [mart.]
49. Butalbital [who-dd]
50. Schembl79820
51. Divk1c_000970
52. Gtpl7138
53. Butalbital [orange Book]
54. Dtxsid6022711
55. Schembl21065545
56. Bucet Component Butalbital
57. Esgic Component Butalbital
58. Hms503a21
59. Kbio1_000970
60. Triad Component Butalbital
61. Butalbital [usp Impurity]
62. Butalbital Ciii [usp-rs]
63. Axotal Component Butalbital
64. Bancap Component Butalbital
65. Femcet Component Butalbital
66. Ninds_000970
67. Tencon Component Butalbital
68. Butalbital [usp Monograph]
69. Hms3713k20
70. Anoquan Component Butalbital
71. Butalbital 1.0 Mg/ml In Methanol
72. Butapap Component Butalbital
73. Sedapap Component Butalbital
74. Fioricet Component Butalbital
75. Fiorinal Component Butalbital
76. Triaprin Component Butalbital
77. Zinc3830347
78. Butalbital Component Of Bucet
79. Butalbital Component Of Esgic
80. Butalbital Component Of Triad
81. 5-(2-methylpropyl)-5-(prop-2-en-1-yl)-1,3-diazinane-2,4,6-trione
82. Lanorinal Component Butalbital
83. Phrenilin Component Butalbital
84. Butalbital Component Of Axotal
85. Butalbital Component Of Bancap
86. Butalbital Component Of Femcet
87. Butalbital Component Of Tencon
88. 5-(2-methylpropyl)-5-(prop-2-en-1-yl)pyrimidine-2,4,6(1h,3h,5h)-trione
89. 5-(2-methylpropyl)-5-prop-2-en-1-ylpyrimidine-2,4,6(1h,3h,5h)-trione
90. Akos003398680
91. Akos015894376
92. Butalbital Component Of Anoquan
93. Butalbital Component Of Butapap
94. Butalbital Component Of Sedapap
95. Butalbital Component Of Fioricet
96. Butalbital Component Of Fiorinal
97. Butalbital Component Of Triaprin
98. Ccg-220459
99. Db00241
100. Butalbital Component Of Lanorinal
101. Butalbital Component Of Phrenilin
102. Idi1_000970
103. Ncgc00344559-01
104. 5-allyl-5-isobutyl-barbitursa Currencyure
105. Butalbital Component Of Medigesic Plus
106. D03182
107. Sr-01000872704
108. Q1606543
109. Sr-01000872704-1
110. W-109270
111. Brd-k71350836-001-01-6
112. 5-allyl-5-isobutylpyrimidine-2,4,6(1h,3h,5h)-trione
113. Butalbital, United States Pharmacopeia (usp) Reference Standard
114. 5-(2-methylpropyl)-5-(2-propen-1-l)-2,4,6(1h,3h,5h)-pyrimidinetrione
115. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-(2-methylpropyl)-5-(2-propen-1-yl)-
116. 4,6-dihydroxy-5-(2-methylpropyl)-5-(prop-2-en-1-yl)-2,5-dihydropyrimidin-2-one
117. Butalbital Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 224.26 g/mol |
|---|---|
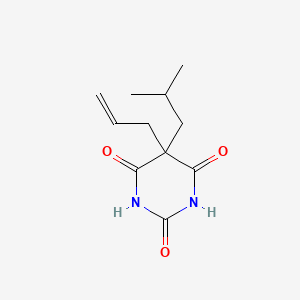
| Molecular Formula | C11H16N2O3 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 224.11609238 g/mol |
| Monoisotopic Mass | 224.11609238 g/mol |
| Topological Polar Surface Area | 75.3 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 327 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Analgesics containing butalbital compounded with aspirin, acetaminophen, and/or caffeine are widely used for the treatment of migraine and tension-type headache. The butalbital-containing compounds are efficacious in placebo-controlled trials among patients with episodic tension-type headaches. Despite their frequent clinical use for migraine, they have not been studied in placebo-controlled trials among patients with migraine. Barbiturates can produce intoxication, hangover, tolerance, dependence, and toxicity. Butalbital can result in intoxication that is clinically indistinguishable from that produced by alcohol. Butalbital-containing analgesics can produce drug-induced headache in addition to tolerance and dependence. Higher doses can produce withdrawal syndromes after discontinuation. Butalbital-containing analgesics may be effective as backup medications or when other medications are ineffective or cannot be used. Because of concerns about overuse, medication-overuse headache, and withdrawal, their use should be limited and carefully monitored.
PMID:11903523 Silberstein SD, McCrory DC; Headache 41 (10): 953-67 (2001)
When patients who have frequent, disabling migraines take medications to relieve their symptoms, they run the risk that the attacks will increase in frequency to daily or near-daily as a rebound effect comes into play. This pattern, called medication overuse headache, is more likely to happen with butalbital and opioids than with migraine-specific drugs, as partial responses lead to recurrence, repeat dosing, and, eventually, overuse. Breaking the cycle involves weaning the patient from the overused medications, setting up a preventive regimen, and setting strict limits on the use of medications to relieve acute symptoms.
PMID:20360117 Tepper SJ, Tepper DE; Cleve Clin J Med 77 (4): 236-42 (2010)
Butalbital is habit-forming and potentially abusable. Consequently, the extended use of this product is not recommended
US Natl Inst Health; DailyMed. Current Medication Information for BUTALBITAL, ACETOMINOPHEN AND CAFFEINE tablet (December 2009). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15032
Butalbital is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function
US Natl Inst Health; DailyMed. Current Medication Information for BUTALBITAL, ACETOMINOPHEN AND CAFFEINE tablet (December 2009). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15032
Indicated for the management of the symptom complex of tension (or muscle contraction) headache, when other non-opioid analgesics and alternative treatments are inadequate, in various combinations with acetaminophen, aspirin, caffeine, and codeine .
Butalbital is a short to intermediate-acting barbiturate that reversibly depresses the activity of excitable tissues, including the central nervous system in a nonselective manner. Barbiturates exhibit muscle-relaxing and anti-anxiety properties and they are capable of producing all levels of CNS mood alteration from excitation to mild sedation, hypnosis, and deep coma. The sedative dose of butalbital in nontolerant individuals is 5-100 mg and the hypnotic dose is 100-200 mg. Pain perception and reaction are relatively unimpaired until the moment of unconsciousness. In some cases, an unwanted paradoxical response of excitement may be observed instead of sedation with barbiturate treatment, which may be due to their depressant effects on inhibitory centers of the CNS. At sufficiently high therapeutic doses, barbiturates induce anesthesia; however, barbiturates are reported to lose their effectiveness for sleep induction and sleep maintenance after 2 weeks. Barbiturates are habit-forming; they can produce tolerance and both dependence and addiction, which is partly explained by decreased drug concentration at the site of action due to enhanced drug metabolism by induced enzymes, or to cellular adaptive changes. In addition, butalbital may lead to analgesic overuse headache. While butalbital is expected to mediate similar actions as other members of the barbiturate drug class, the effects of butalbital in isolation are not well understood. It is suggested that butalbital is added in combination products to antagonize the unwanted central stimulating effect of stimulatory ingredients such as caffeine. Butalbital may decrease blood pressure and heart rate when administered at sedative and hypnotic doses.
Absorption
Butalbital gets readily and rapidly absorbed from the gastrointestinal tract. The time to reach the peak plasma concentrations is reported to be approximately 2 hours. Typical blood concentrations of butalbital peaked at 2.1 mg/L and declined to 1.5 mg/L at 24 hr. Plasma concentrations of 10 to 20 g/mL have been associated with toxicity; coma and fatalities have occurred with concentrations of 25 to 30 g/mL.
Route of Elimination
Butalbital predominantly undergoes renal elimination with 59 to 88% of the total dose administered being excreted from the kidneys as unchanged parent drug or metabolites. Urinary excretion products included parent drug (about 3.6% of the dose), 5-isobutyl-5-(2,3-dihydroxypropyl) barbituric acid (about 24% of the dose), 5-allyl-5(3-hydroxy-2-methyl-1-propyl) barbituric acid (about 4.8%), products with the barbituric acid ring hydrolyzed with excretion of urea (about 14% of the dose), as well as unidentified materials. Of the material excreted in the urine, 32% is conjugated. Elimination is not complete within 24 hours, and the drug accumulates with frequent administration.
Volume of Distribution
The volume of distribution of butalbital is reported to be approximately 0.8L/kg. Butalbital is expected to distribute to most of the tissues in the body, including the mamillary glands and placenta. The plasma-to-blood concentration ratio was almost unity indicating that there is no preferential distribution of butalbital into either plasma or blood cells.
Clearance
There is limited data on the clearance of butalbital.
Butalbital is well absorbed from the gastrointestinal tract and is expected to distribute to most tissues in the body. Barbiturates in general may appear in breast milk and readily cross the placental barrier. They are bound to plasma and tissue proteins to a varying degree and binding increases directly as a function of lipid solubility.
US Natl Inst Health; DailyMed. Current Medication Information for BUTALBITAL, ACETOMINOPHEN AND CAFFEINE tablet (December 2009). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15032
Blood and plasma concentrations of butalbital were determined in a small group of healthy volunteers after single oral doses of 100 mg of butalbital. Butalbital was quantitated by high-performance liquid chromatography with ultraviolet detection. Typical blood concentrations of butalbital peaked at 2.1 mg/L and declined to 1.5 mg/L at 24 hr.
PMID:3244271 Drost ML, Walter L; J Anal Toxicol 12 (6): 322-4 (1988)
The in vitro plasma protein binding of butalbital is 45% over the concentration range of 0.5-20 ug/mL. This falls within the range of plasma protein binding (20%-45%) reported with other barbiturates such as phenobarbital, pentobarbital, and secobarbital sodium. The plasma-to-blood concentration ratio was almost unity, indicating that there is no preferential distribution of butalbital into either plasma or blood cells.
US Natl Inst Health; DailyMed. Current Medication Information for BUTALBITAL, ACETOMINOPHEN AND CAFFEINE tablet (December 2009). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15032
Elimination of butalbital is primarily via the kidney (59% to 88% of the dose) as unchanged drug or metabolites.
US Natl Inst Health; DailyMed. Current Medication Information for BUTALBITAL, ACETOMINOPHEN AND CAFFEINE tablet (December 2009). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15032
Butalbital is expected to undergo nearly complete hepatic metabolism. It primarily undergoes C5 oxidation to form 5-isobutyl-5-(2,3-dihydroxypropyl) barbituric acid, which is the major metabolite. Butalbital may also undergo omega-hydroxylation to form 5-allyl-5(3-hydroxy-2-methyl-1-propyl) barbituric acid.
Urinary excretion products include parent drug (about 3.6% of the dose), 5-isobutyl-5-(2, 3-dihydroxypropyl) barbituric acid (about 24% of the dose), 5-allyl-5(3-hydroxy-2-methyl-1-propyl) barbituric acid (about 4.8% of the dose), products with the barbituric acid ring hydrolyzed with excretion of urea (about 14% of the dose), as well as unidentified materials. Of the material excreted in the urine, 32% is conjugated.
US Natl Inst Health; DailyMed. Current Medication Information for BUTALBITAL, ACETOMINOPHEN AND CAFFEINE tablet (December 2009). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15032
Butalbital, 5-allyl-5-isobutylbarbituric acid, labeled in the 2-position with 14C, was administered to dogs. Ninety-two percent of the radioactivity of the dose was excreted in the urine. The drug and three major urinary metabolites were identified in the urinary excretion of the dog. The major metabolite was 5-isobutyl-5-(2,3-dihydroxypropyl)barbituric acid, which accounted for 50.2% of the dose. Smaller amounts of the unchanged drug (2.6% of the dose) and urea (8.6% of the dose) were present. 5-Allyl-5-(3-hydroxy-2-methyl-1-propyl)barbituric acid, formed by omega-hydroxylation, accounted for 10.1% of the dose; the optical rotation of the 1,3-diethyl derivative was [alpha]D20 = +10.5. Five minor and unidentified metabolities accounted for an additional 10.7% of the dose. A total of 82.2% of the dose was accounted for.
PMID:6105059 Dain JG et al; Drug Metab Dispos 8 (4): 247-52 (1980)
The plasma half-life is about 35 hours. In a study of 5 healthy volunteers receiving 100 mg butalbital in combination with aspirin and caffeine, the mean plasma elimination half-life of butalbital was 61 hours, with the range of 35 to 88 hours.
Elimination of butalbital is primarily via the kidney (59% to 88% of the dose) as unchanged drug or metabolites. The plasma half-life is about 35 hours.
US Natl Inst Health; DailyMed. Current Medication Information for BUTALBITAL, ACETOMINOPHEN AND CAFFEINE tablet (December 2009). Available from, as of November 17, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=15032
Blood and plasma concentrations of butalbital were determined in a small group of healthy volunteers after single oral doses of 100 mg of butalbital. ... The half-lives in blood were between 35 and 87.5 hr with a mean of 61 hr.
PMID:3244271 Drost ML, Walter L; J Anal Toxicol 12 (6): 322-4 (1988)
Butalbital is a CNS depressant that suppresses neuronal excitability, impulse conduction, and the release of neurotransmitters, similar to actions of other barbiturates. Barbiturates primarily mediate suppressive actions on polysynaptic neuronal responses by diminishing facilitation while enhancing inhibition. Inhibition occurs at GABAergic synapses that express GABA-A receptors, which are transmembrane chloride ion channels that bind an inhibitory neurotransmitter GABA, barbiturates, benzodiazepines, neurosteroids, and ethanol. Upon activation, GABA-A receptors allow Cl- influx and K+ efflux into the postjunctional terminal, resulting in inhibition of the postsynaptic neuron. It is suggested that barbiturates, including butalbital, enhances GABA binding to the GABA-A receptors by binding to the +/ interface in the intracellular domain (ICD) as an allosteric modulator. Additionally, barbiturates promote benzodiazepine binding to the receptor. Barbiturates potentiate GABA-induced increases in chloride conductance and depress voltage-activated calcium currents while prolonging the duration of GABA-induced chloride channel opening. Butalbital may also inhibit the excitatory effects mediated by AMPA receptors by reducing glutamate-induced depolarizations of the receptor. It is also proposed that barbiturates and opioids may potentiate the analgesic effects of each other when co-administered, although there are inconsistencies across preclinical data.
The effects of butalbital (30, 100, and 1000 ug/kg) on the number of cells expressing c-fos-like immunoreactivity (c-fos-LI), a marker of neuronal activation, within lamina I, IIo of the trigeminal nucleus caudalis and the nucleus of the solitary tract 2 hours after the intracisternal injection of capsaicin (0.1 mL; 15.25 mg/mL) or vehicle in urethane-anesthetized guinea pigs (N = 45) /was examined/. Robust c-fos-LI was observed within nuclei of cells in the trigeminal nucleus caudalis after capsaicin (329 +/- 35). Butalbital dose-dependently reduced the number of labeled cells to a maximum of 66% (1000 micrograms/kg intraperitoneally [i.p.], P < .01) in lamina I, IIo but not within area postrema, medial reticular nucleus, or the nucleus of the solitary tract. Pretreatment with bicuculline (30 micrograms/kg i.p.) blocked the effect of butalbital, thereby suggesting the importance of the GABAA receptor to activation involved in the transmission of nociceptive information. Our studies suggest the possibility that GABAA receptors might provide an important therapeutic target in migraine and related headache disorders.
PMID:11279945 Cutrer FM et al; Headache 39 (10): 697-704 (1999)








