



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
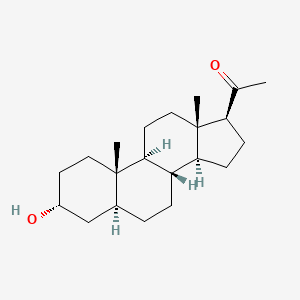

1. 3 Alpha Hydroxy 5 Alpha Pregnan 20 One
2. 3 Alpha Hydroxy 5 Beta Pregnan 20 One
3. 3 Alpha, 5 Beta Tetrahydroprogesterone
4. 3 Alpha, 5 Beta-tetrahydroprogesterone
5. 3 Alpha-hydroxy-5 Alpha-pregnan-20-one
6. 3 Alpha-hydroxy-5 Beta-pregnan-20-one
7. 3 Hydroxypregnan 20 One
8. 3-hydroxypregnan-20-one
9. 3beta Hydroxy 5alpha Pregnan 20 One
10. 3beta-hydroxy-5alpha-pregnan-20-one
11. Allopregnan 3 Beta Ol 20 One
12. Allopregnan-3 Beta-ol-20-one
13. Alpha-hydroxy-5 Alpha-pregnan-20-one, 3
14. Alpha-hydroxy-5 Beta-pregnan-20-one, 3
15. Alpha-pregnan-20-one, 3 Alpha-hydroxy-5
16. Beta-ol-20-one, Allopregnan-3
17. Beta-pregnan-20-one, 3 Alpha-hydroxy-5
18. Eltanolone
19. Epipregnanolone
20. Pregnan 3alpha Ol 20 One
21. Pregnan-3alpha-ol-20-one
22. Pregnanolone
23. Pregnanolone, (3alpha)-isomer
24. Pregnanolone, (3alpha, 5beta, 17-alpha)-isomer
25. Pregnanolone, (3alpha,5alpha)-isomer
26. Pregnanolone, (3alpha,5beta)-isomer
27. Pregnanolone, (3beta)-isomer
28. Pregnanolone, (3beta, 5alpha)-isomer
29. Pregnanolone, (3beta, 5alpha, 17alpha)-isomer
30. Pregnanolone, (3beta, 5alpha, 8alpha, 17beta)-isomer
31. Pregnanolone, (3beta, 5beta)-isomer
32. Pregnanolone, (3beta, 5beta, 17alpha)-isomer
33. Pregnanolone, (3beta, 5beta,14beta)-isomer
34. Pregnanolone, (5alpha)-isomer
35. Sepranolone
1. 516-54-1
2. Brexanolone
3. Allopregnan-3alpha-ol-20-one
4. Allotetrahydroprogesterone
5. Sage-547
6. Zulresso
7. 3alpha-hydroxy-5alpha-pregnan-20-one
8. 3alpha-oh Dhp
9. 3alpha,5alpha-thp
10. Sge-102
11. 3a,5a-thp
12. (3alpha,5alpha)-3-hydroxypregnan-20-one
13. Brexanolone [usan]
14. 3alpha-hydroxy-5alpha-dihydroprogesterone
15. 3a-hydroxy-5a-pregnan-20-one
16. S39xz5qv8y
17. Chembl207538
18. Pregnan-20-one, 3-hydroxy-, (3a,5a)-
19. Chebi:50169
20. 3alpha,5alpha-tetrahydroprogesterone
21. 1-[(3r,5s,8r,9s,10s,13s,14s,17s)-3-hydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]ethanone
22. 1-((3r,5s,8r,9s,10s,13s,14s,17s)-3-hydroxy-10,13-dimethylhexadecahydro-1h-cyclopenta[a]phenanthren-17-yl)ethan-1-one
23. Allopregnan-3.alpha.-ol-20-one
24. C21h34o2
25. 3-alpha,5-alpha-pregnanolone
26. Sage547
27. (3
28. A)-allopregnanolone
29. Unii-s39xz5qv8y
30. 3-alpha,5-alpha-tetrahydroprogesterone
31. Allopregnan-3a-ol-20-one
32. Brexanolona
33. Brexanolonum
34. Brn 3211363
35. Allopregnalone
36. 3-alpha-hydroxy-5-alpha-pregnan-20-one
37. 3alpha-hydroxy-5alpha-pregnane-20-one
38. (3?,5?)-3-hydroxy-pregnan-20-one
39. Ncgc00163287-01
40. Zulresso (tn)
41. 3a,5a-pregnanolone
42. (3a)-allopregnanolone
43. 3a,5a-thprog
44. 5-alpha-pregnan-20-one, 3-alpha-hydroxy-
45. Brexanolone [inn]
46. (3alpha)-allopregnanolone
47. 3-a-tetrahydroprogesterone
48. Brexanolone (usan/inn)
49. Pregnan-20-one, 3-hydroxy-, (3.alpha.,5.alpha.)-
50. Biomol-nt_000266
51. Dsstox_cid_26342
52. Dsstox_rid_81546
53. 3a,5a-tetrahydroprogesterone
54. Bidd:pxr0069
55. Dsstox_gsid_46342
56. 5a-pregnan-3a-ol-20-one
57. Brexanolone [who-dd]
58. 3-alpha-tetrahydroprogesterone
59. 4-08-00-00664 (beilstein Handbook Reference)
60. 5a-pregnane-3a-ol-20-one
61. Schembl588060
62. Pregnan-20-one, 3-hydroxy-, (3-alpha,5-alpha)-
63. Bpbio1_000728
64. Gtpl4108
65. 3a-hydroxy-5a-pregnane-20-one
66. Brexanolone [orange Book]
67. 3a-hydroxy-5a-dihydroprogesterone
68. 5
69. A-pregnan-3
70. A-ol-20-one
71. 5alpha Pregnan 3alpha Ol 20 One
72. Dtxsid901016239
73. Hms3886d05
74. 5alpha-pregnane-3alpha-ol-20-one
75. Amy16827
76. Ex-a1195
77. Zinc4081043
78. Tox21_112042
79. (3a,5a)-3-hydroxypregnan-20-one
80. Bdbm50191342
81. Lmst02030156
82. S5805
83. (+)-3a-hydroxy-5a-pregnan-20-one
84. Akos024285177
85. Pregnane-3alpha-ol-20-one, 5alpha-
86. Cs-6401
87. Db11859
88. 3
89. A,5
90. A-thp;sage-547;brexanolone
91. Ncgc00163287-02
92. 5alpha-pregnan-3alpha-ol-20-one, Solid
93. Ac-30353
94. As-76408
95. Cas-128-20-1
96. (3alpha,5alpha)-3-hydroxy-pregnan-20-one
97. Hy-101107
98. B7456
99. Allopregnan-3.alpha.-ol-20-one [mi]
100. D11149
101. 516a541
102. 3.alpha.-hydroxy-5.alpha.-pregnan-20-one
103. Brd-k18172896-001-01-7
104. Q28487680
105. (+)-3.alpha.-hydroxy-5.alpha.-pregnan-20-one
106. 8e5d696a-eebc-495d-a8c9-98fc9a593150
| Molecular Weight | 318.5 g/mol |
|---|---|
| Molecular Formula | C21H34O2 |
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 318.255880323 g/mol |
| Monoisotopic Mass | 318.255880323 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 500 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Brexanolone is a synthetic neuroactive steroid gamma-aminobutyric acid A (GABA(a)) receptor positive modulator indicated for the treatment of postpartum depression (PPD) in adult women.
FDA Label
Treatment of postpartum depression
Brexanolone potentiated GABA-mediated currents from recombinant human GABA(a) receptors in mammalian cells expressing 122 receptor subunits, 43 receptor subunits, and 63 receptor subunits. Moreover, it was determined during a Phase 1 randomized, placebo and positive-controlled, double-blind, three-period crossover thorough QT study in 30 healthy adult subjects that brexanolone use did not prolong the QT interval to any clinically relevant extent when administered at 1.9-times the exposure occurring at the highest recommended infusion rate (90 mcg/kg/hour).
Anesthetics
Agents capable of inducing a total or partial loss of sensation, especially tactile sensation and pain. They may act to induce general ANESTHESIA, in which an unconscious state is achieved, or may act locally to induce numbness or lack of sensation at a targeted site. (See all compounds classified as Anesthetics.)
Absorption
It has been determined that brexanolone has a low oral bioavailability of approximately <5% in adults, which suggests infant exposure would also be expected to be low.
Route of Elimination
Following the administration of radiolabeled brexanolone, it was observed that 47% of the administrated dose was recovered largely as metabolites in the feces and 42% in urine, where less than 1% as recovered as unchanged brexanolone.
Volume of Distribution
The volume of distribution documented for brexanolone is approximately 3 L/kg, a value which suggests relatively extensive distribution into tissues.
Clearance
The total plasma clearance determined for brexanolone is approximately 1 L/h/kg.
Brexanolone is extensively metabolized by non-cytochrome (CYP) based pathways by way of three main routes - keto-reduction (via aldo-keto reductases), glucuronidation (via UDP-glucuronosyltransferases), and sulfation (via sulfotransferases). Three predominant circulating metabolites result from such metabolic pathways and they are all pharmacologically inactive and ultimately do not contribute to the overall efficacy of the medication.
The terminal half-life observed for brexanolone is approximately 9 hours.
Brexanolone is a neuroactive steroid that occurs naturally (referred to as natural allopregnanolone) in the body when the female sex hormone progesterone is metabolized. This steroid compound is also believed to exhibit activity as a barbiturate-like, positive allosteric modulator of both synaptic and extrasynaptic GABA(a) receptors. In doing so, brexanolone can enhance the activity of GABA at such receptors by having GABA(a) receptor calcium channels open more often and for longer periods of time. Furthermore, it is believed that brexanolone elicits such action on GABA(a) receptors at a binding site that is distinct from those associated with benzodiazepines. Concurrently, GABA is considered the principal inhibitory neurotransmitter in the human body. When GABA binds to GABA(a) receptors found in neuron synapses, chloride ions are conducted across neuron cell membranes via an ion channel in the receptors. With enough chloride ions conducted, the local, associated neuron membrane potentials are hyperpolarized - making it more difficult or less likely for action potentials to fire, ultimately resulting in less excitation of the neurons, like those involved in neuronal pathways that may be in part responsible for eliciting certain traits of PPD like stress, anxiety, etc. Postpartum depression (PPD) is a mood disorder that can affect women after childbirth. Women with PPD experience feelings of extreme sadness, anxiety, and exhaustion that can make it difficult or even dangerous for them to perform various daily activities or care for themselves or for others, including newborn. Although the exact pathophysiology of PPD remains unknown, it is believed that altered profiles and rapid, unpredictable fluctuations in the blood concentrations of neuroactive steroids like endogenous brexanolone (among others), GABA, and GABA receptors occur in women who are at risk of PPD after childbirth. In particular, within the context of PPD, it is proposed that endogenous brexanolone levels can quickly drop or fluctuate variedly after childbirth and that GABA(a) receptor levels and expression are decreased and down-regulated throughout pregnancy. Such fluctuations and decreases may consequently leave women susceptible to the possibility of PPD. As a medication, synthetic brexanolone can subsequently facilitate a return of positive allosteric modulator GABA(a) modulation while GABA(a) receptor levels and expression gradually return to normal in the time following postpartum. As such, studies suggest the potential for the development of brexanolone as a new mechanism for treatment of PPD that is directly related to the underlying pathophysiology as opposed to many other antidepressant medications whose pharmacological actions are usually entirely unrelated.


