



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
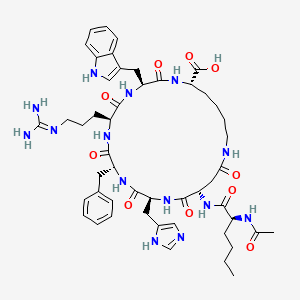

1. Pt-141
2. Vyleesi
1. 189691-06-3
2. Pt-141
3. Pt-141 Free Base
4. 6y24o4f92s
5. Vyleesi (tn)
6. (3s,6s,9r,12s,15s,23s)-15-[[(2s)-2-acetamidohexanoyl]amino]-9-benzyl-6-[3-(diaminomethylideneamino)propyl]-12-(1h-imidazol-5-ylmethyl)-3-(1h-indol-3-ylmethyl)-2,5,8,11,14,17-hexaoxo-1,4,7,10,13,18-hexazacyclotricosane-23-carboxylic Acid
7. (3s,6s,9r,12s,15s,23s)-12-((1h-imidazol-5-yl)methyl)-3-((1h-indol-3-yl)methyl)-15-((s)-2-acetamidohexanamido)-9-benzyl-6-(3-guanidinopropyl)-2,5,8,11,14,17-hexaoxo-1,4,7,10,13,18-hexaazacyclotricosane-23-carboxylic Acid
8. Bremelanotide [usan:inn]
9. Unii-6y24o4f92s
10. Bremelanotide, Pt-141
11. Bremelanotide [mi]
12. Bremelanotide [inn]
13. Pt-141 (bremelanotide)
14. Bremelanotide (usan/inn)
15. Bremelanotide [usan]
16. Bremelanotide [who-dd]
17. Chembl2070241
18. Schembl13574795
19. Schembl20337333
20. Gtpl10408
21. Dtxsid40893711
22. Chebi:177849
23. Pt141
24. 189691-06-3 (free Base)
25. Bdbm50389769
26. Akos005145807
27. Db11653
28. Hs-2024
29. Hy-18678
30. Cs-0013839
31. D06569
32. Q415353
33. Q-200747
34. (1z,3s,4z,6s,7z,9r,10e,12s,13z,15s,17e,23s)-12-((1h-imidazol-5-yl)methyl)-3-((1h-indol-3-yl)methyl)-9-benzyl-6-(3-guanidinopropyl)-2,5,8,11,14,17-hexahydroxy-15-(((s,z)-1-hydroxy-2-(((z)-1-hydroxyethylidene)amino)hexylidene)amino)-1,4,7,10,13,18-hexaazacyclotricosa-1,4,7,10,13,17-hexaene-23-carboxylic Acid
35. L-lysine, N-acetyl-l-norleucyl-l-.alpha.-aspartyl-l-histidyl-d-phenylalanyl-l-arginyl-l-tryptophyl-, (2->7)-lactam
36. L-lysine, N-acetyl-l-norleucyl-l-alpha-aspartyl-l-histidyl-d-phenylalanyl-l-arginyl-l-tryptophyl-, (2->7)-lactam
37. N-acetyl-l-2-aminohexanoyl-l-.alpha.-aspartyl-l-histidyl-d-phenylalanyl-l-arginyl-l-tryptophyl-l-lysine-(2->7)-lactam
38. N-acetyl-l-2-aminohexanoyl-l-alpha-aspartyl-l-histidyl-d-phenylalanyl-l-arginyl-l-tryptophyl-l-lysine-(2->7)-lactam
| Molecular Weight | 1025.2 g/mol |
|---|---|
| Molecular Formula | C50H68N14O10 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 13 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 17 |
| Exact Mass | 1024.52428442 g/mol |
| Monoisotopic Mass | 1024.52428442 g/mol |
| Topological Polar Surface Area | 379 Ų |
| Heavy Atom Count | 74 |
| Formal Charge | 0 |
| Complexity | 1950 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Bremelanotide is indicated to treat premenopausal women with hypoactive sexual desire disorder that is not due to a medical or psychiatric condition, problems with the relationship, or the effects of a medication or drug.
Bremelanotide is a melanocortin receptor agonist injected 45 minutes before anticipated sexual activity. Agonism of the melanocortin receptor MC1R also leads to increased melanin expression. Patients taking bremelanotide may also experience nausea, headache, and vomiting.
G - Genito urinary system and sex hormones
G02 - Other gynecologicals
G02C - Other gynecologicals
G02CX - Other gynecologicals
G02CX05 - Bremelanotide
Absorption
Bremelanotide has a Tmax or 1.0 hour (0.5-1.0 hours) and is 100% bioavailable. The Cmax is 72.8ng/mL and the AUC is 276hr\*ng/mL.
Route of Elimination
64.8% of a radiolabelled dose is excreted in the urine and 22.8% of the dose is recovered in the feces.
Volume of Distribution
The mean volume of distribution of bremelanotide is 25.05.8L.
Clearance
The mean clearance of bremelanotide is 6.51.0L/hr.
Bremelanotide is a 7 amino acid and so its metabolism consists of multiple hydrolysis reactions.
The half life of bremelanotide is 2.7 hours (1.9-4.0 hours).
Bremelanotide is an agonist of many melanocortin receptors which in order of potency are MC1R, MC4R, MC3R, MC5R, and MC2R. The mechanism by which agonism of these receptors translates to an improvement in hypoactive sexual desire disorder is currently unknown, however MC4R receptors are present in many areas of the central nervous system. MC3R and MC4R are found in the hypothalamus and are involved in food intake and energy homeostasis. One theory is that bremelanotide stimulates dopamine in the medial preoptic area, which is involved in the sexual behaviour of a number of organisms.


