


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
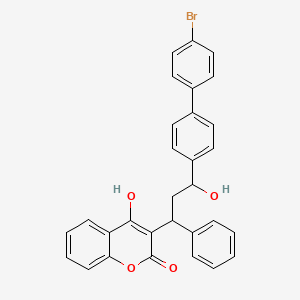

1. Bromodialone
1. 28772-56-7
2. Broprodifacoum
3. Bromadiolon
4. Bromatrol
5. Contrac
6. Ratimus
7. Boldo
8. Temus
9. Sup'operats
10. Super-rozol
11. Boot Hill
12. Canadien 2000
13. Maki
14. Bromone
15. Super-caid
16. Chebi:81855
17. Lm-637
18. 3-[3-[4-(4-bromophenyl)phenyl]-3-hydroxy-1-phenylpropyl]-4-hydroxychromen-2-one
19. 3-(3-(4'-bromo(1,1'-biphenyl)-4-yl)3-hydroxy-1-phenylpropyl)-4-hydroxy-2h-1-benzopyran-2-one
20. 2h-1-benzopyran-2-one, 3-[3-(4'-bromo[1,1'-biphenyl]-4-yl)-3-hydroxy-1-phenylpropyl]-4-hydroxy-
21. Bromore
22. Contrax
23. Topidion
24. Eradic
25. Rafix
26. Super-cald
27. (hydroxy-4 Coumarinyl 3)-3 Phenyl-3 (bromo-4 Biphenylyl-4)-1 Propanol-1
28. Coumarin, 3-(3-(4'-bromo-1,1'-biphenyl-4-yl)-3-hydroxy-1-phenylpropyl)-4-hydroxy-
29. Dsstox_cid_12589
30. Dsstox_rid_78997
31. Dsstox_gsid_32589
32. 2h-1-benzopyran-2-one, 3-(3-(4'-bromo(1,1'-biphenyl)-4-yl)-3-hydroxy-1-phenylpropyl)-4-hydroxy-
33. Bromodiolone
34. Caswell No. 486ab
35. Bromadiolone [bsi:iso]
36. Broprodifacoum [south Africa]
37. Cas-28772-56-7
38. Hsdb 6458
39. Einecs 249-205-9
40. Epa Pesticide Chemical Code 112001
41. Brn 1335336
42. Bromadialone
43. Tamogam
44. Slaymor (salt/mix)
45. 3-(4-hydroxycoumarin-3-yl)-3-phenyl-1-(4-bromobiphenyl)propan-1-ol
46. 3-(alpha-(p-bromophenyl)-beta-hydroxyphenethyl)benzyl-4-hydroxycoumarin
47. 3-(3-(4'-bromobiphenyl-4-yl)-3-hydroxy-1-phenylpropyl)-4-hydroxycoumarin
48. 3-(4-hydroxy-2-oxochromen-3-yl)-3-phenyl-1-(4-bromobiphenyl)propan-1-ol
49. 3-(alpha-(p-(p-bromophenyl)-beta-hydroxyphenethyl)benzyl)-4-hydroxycoumarin
50. Coumarin, 3-(alpha-(p-(p-bromophenyl)-beta-hydroxyphenethyl)benzyl)-4-hydroxy-
51. (hydroxy-4 Coumarinyl 3)-3 Phenyl-3 (bromo-4 Biphenylyl-4)-1 Propanol-1 [french]
52. (hydroxy-4-coumarinyl-3)-3-phenyl-3-(bromo-4-biphenylyl-4)-1-propanol-1 [french]
53. Rentokil Deadline (salt/mix)
54. Schembl433993
55. Schembl5933914
56. Chembl1165553
57. Dtxsid9032589
58. Tox21_112561
59. Tox21_301920
60. (hydroxy-4-coumarinyl-3)-3-phenyl-3-(bromo-4-biphenylyl-4)-1-propanol-1
61. Akos015907923
62. Akos030254788
63. Tox21_112561_1
64. 3-(3-(4'-bromo(1,1'-biphenyl)-4-yl)-3-hydroxy-1-phenylpropyl)-4-hydroxy-2-benzopyrone
65. 3-[3-[4-(4-bromophenyl)phenyl]-3-hydroxy-1-phenylpropyl]-2-hydroxychromen-4-one
66. Ncgc00163939-01
67. Ncgc00163939-02
68. Ncgc00255356-01
69. Ac-12416
70. Bromadiolone 100 Microg/ml In Acetonitrile
71. Db-047454
72. Bromadiolon, Pestanal(r), Analytical Standard
73. C18596
74. 772b567
75. A819585
76. Q423091
77. J-017254
78. 3-(.alpha.-(p-(p-bromophenyl)-.beta.-hydroxyphenethyl)benzyl)-4-hydroxycoumarin
79. 3-(1-phenyl-3-hydroxy-3-(4-(4-bromophenyl)pheny)propyl)-4-hydroxy-coumarin
80. 3-(3-(4'-bromo-(1,1'-biphenyl)-4-yl)-3-hydroxy-1-phenylpropyl)-4-hydroxy-coumarin
81. 3-(3-(4'-bromobiphenyl-4-yl)-3-hydroxy-1-phenylpropyl)-4-hydroxy-2h-chromen-2-one
82. 3-[3-[4-(4-bromophenyl)phenyl]-3-hydroxy-1-phenylpropyl]-2-hydroxy-1-benzopyran-4-one
83. 3-[3-[4-(4-bromophenyl)phenyl]-3-oxidanyl-1-phenyl-propyl]-2-oxidanyl-chromen-4-one
84. Coumarin, 3-(.alpha.-(p-(p-bromophenyl)-.beta.-hydroxyphenethyl)benzyl)-4-hydroxy-
85. 3-[3-(4'-bromo[1,1'-biphenyl]-4-yl)-3-hydroxy-1-phenylpropyl]-4-hydroxy-2h-chromen-2-one
| Molecular Weight | 527.4 g/mol |
|---|---|
| Molecular Formula | C30H23BrO4 |
| XLogP3 | 6.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 526.07797 g/mol |
| Monoisotopic Mass | 526.07797 g/mol |
| Topological Polar Surface Area | 66.8 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 744 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anticoagulants
Agents that prevent BLOOD CLOTTING. (See all compounds classified as Anticoagulants.)
Rodenticides
Substances used to destroy or inhibit the action of rats, mice, or other rodents. (See all compounds classified as Rodenticides.)
Rats (Rattus norvegicus) dosed orally with the rodenticide bromadiolone (0.8 and 3 mg/kg) were sacrificed in groups of 4 rats at various times up to 97 hr after administration. Bromadiolone was assayed in plasma, liver and kidney. ... The compound disappeared slowly from the organism with a half-life of 25.7 hr for the 0.8 mg/kg dose and 57.5 hr for the 3 mg/kg. Concentrations in liver were rapidly established and were 14- to 46-fold higher than plasma concentrations. /Liver concentrations were about 1.5 ug/g/ 97 hr after 8 mg/kg dose. Bromadiolone levels in kidney were slightly higher than those observed in plasma with a longer half-life.
PMID:3444841 Kamil N; Pharmacol Res Commun 19 (11): 767-76 (1987)
Groups of male Sprague-Dawley rats received a single dose (0.2 mg/kg bw) of brodifacoum, bromadiolone, or flocoumafen by gavage. A control group consisting of 9 male rats which received nothing was also included in the study. .... During the first 28 days after dosing, the decline of the liver concns of bromadiolone and flocoumafen was faster than that of brodifacoum as indicated by the t1/2's of these 3 chemicals at the first 28 days (brodifacoum: t1/2, 63 days; bromadiolone: t1/2, 17 days; flocoumafen: t1/2, 6 days). The decline of the liver concns of these 3 test chemicals occurred in a "bi-exponential manner". The second t1/2's were estimated to be 282, 318, and 159 days for brodifacoum, bromadiolone, and flocoumafen, respectively. In general, oral admin of any of these 3 chemicals would result in substantial retention of the chemical in the liver for a very long time.
USEPA; Reregistration Eligibility Decision Document - Rodenticide Cluster. Washington, DC: USEPA, Off Pest Prog. USEPA 738-R-98-007, p.38 July 1998. Available from, as of March 17, 2003: https://www.epa.gov/pesticides/reregistration/status.htm
/The goal of this study is/ to assess the rate and extent of ruminal degradation of warfarin, chlorophacinone, and bromadiolone in vitro and determine the oral availability and clinical and hemostatic effects of each anticoagulant rodenticide in adult sheep... Samples of ruminal fluid were incubated with each of the anticoagulants to assess the kinetics of ruminal degradation over 24 hours. To determine the plasma kinetics of the anticoagulants, each /of 3 Texel/ sheep received each of the anticoagulants IV or via a rumen implanted cannula at 2-month intervals (3 rodenticide exposures/sheep). At intervals during a 240- to 360- hour period after treatment, prothrombin time (PT) was measured, plasma anticoagulant concentration was assessed, and clinical signs of rodenticide poisoning were monitored. In plasma and rumen extracts, anticoagulant concentrations were determined via high-performance liquid chromatography. In the rumen extracts, anticoagulants were slightly degraded (< 15%) over 24 hours. In vivo, oral availability of warfarin, chlorophacinone, and bromadiolone was estimated at 79%, 92%, and 88%, respectively. Although maximum PT was 80 seconds after chlorophacinone and bromadiolone treatments, no clinical signs of toxicosis were detected; PT returned to baseline values within 2 weeks. In sheep, warfarin, chlorophacinone, and bromadiolone were not degraded in the rumen but their bioavailabilities were high after oral administration; the kinetics of these compounds in sheep and other mammals are quite similar. These data suggest that the lack of susceptibility of ruminants to these anticoagulant rodenticides cannot be explained by either ruminal degradation or the specific toxicokinetics of these anticoagulants.
PMID:16454646 Berny PJ; Am J Vet Res. 67(2):363-71 (2006).
Warfarin is a widely used anticoagulant in the treatment and prevention of thrombosis, in the treatment for chronic atrial fibrillation, mechanical valves, pulmonary embolism, and dilated cardiomyopathy. It is tasteless and colorless, was used as a poison, and is still marketed as a pesticide against rats and mice. Several long-acting warfarin derivatives-superwarfarin anticoagulants-such as brodifacoum, diphenadione, chlorophacinone, bromadiolone, are used as pesticides and can produce profound and prolonged anticoagulation. Several factors increase the risk of warfarin toxicity. However, polymorphisms in cytochrome P450 genes and drug interactions account for most of the risk for toxicity complications. Each person is unique in their degree of susceptibility to toxic agents. The toxicity interpretation and the health risk of most toxic substances are a subject of uncertainty. Genetically determined low metabolic capacity in an individual can dramatically alter the toxin and metabolite levels from those normally expected, which is crucial for drugs with a narrow therapeutic index, like warfarin. Personalized approaches in interpretation have the potential to remove some of the scientific uncertainties in toxicity cases.
PMID:22069565 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3153177 Piatkov I et al; Toxins (Basel). 2(11):2584-92 (2010).
Anticoagulant resistance in Norway rats, Rattus norvegicus (Berk.), has been suggested to be conferred by mutations in the VKORC1 gene, encoding the target protein of anticoagulant rodenticides. Other factors, e.g. pharmacokinetics, may also contribute to resistance, however. To examine the involvement of pharmacokinetics in bromadiolone resistance in male and female rats, liver expression profiles of seven cytochrome P450 genes from a Danish bromadiolone-resistant rat strain (with an Y139C-VKORC1 mutation) were compared with profiles from an anticoagulant-susceptible strain. In the presence of bromadiolone, the Cyp2e1, Cyp2c13, Cyp3a2 and Cyp3a3 genes were significantly overexpressed, while Cyp2c12 expression was suppressed in resistant female rats compared with susceptible females. Relative to susceptible males, resistant males showed significant overexpression of the Cyp2a1, Cyp2e1, Cyp3a2 and Cyp3a3 genes. On exposure to bromadiolone, females had higher Cyp2e1 expression than males, which possibly explains why female rats are generally more tolerant to anticoagulants than male rats. Results suggest that bromadiolone resistance in a Danish strain of Norway rats involves enhanced anticoagulant metabolism catalyzed by cytochrome P450-2e1, -3a2 and -3a3. This pharmacokinetically based bromadiolone resistance is to some extent sex differentiated, as female resistance furthermore seems to involve overexpression of cytochrome P450-2c13 and suppression of P450-2c12, whereas male resistance appears to involve P450-2a1 overexpression.
PMID:18080289 Markussen MD et al; Pest Manag Sci. 64(3):239-48 (2008).
Bromadiolone, brodifacoum and coumatetralyl were also found in rats as unchanged parent compounds... .
WHO; Environ Health Criteria 175: Anticoagulant Rodenticides p.50 (1995)
Long-acting compounds are metabolized by hepatic cytochrome P-450 isozymes (e.g., CYP3A4). /Long-Acting Rodenticides/
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1498
... Poisoning in a 40-year old female who ... ingested ... equivalent to 8.5 mg bromadiolone (0.17 mg/kg body weight), four days prior to admission. ... The first plasma bromadiolone level (5 days post-ingestion) was 92 ng/mL. Serial measurement of plasma bromadiolone levels confirmed the diagnosis and demonstrated that bromadiolone obeys the elimination kinetic of a two-compartment model with a rapid, fairly steep decline phase (half-life 3.5 days) followed by a slower termination phase (half-life 24 days). ...
PMID:19238731 Lo VM et al; Clin Toxicol (Phila). 46(8):703-10 (2008).
Rats (Rattus norvegicus) dosed orally with the rodenticide bromadiolone (0.8 and 3 mg/kg) were sacrificed in groups of 4 rats at various times up to 97 hr after administration. Bromadiolone was assayed in plasma, liver and kidney. ... The compound disappeared slowly from the organism with a half-life of 25.7 hr for the 0.8 mg/kg dose and 57.5 hr for the 3 mg/kg. ...
PMID:3444841 Kamil N; Pharmacol Res Commun 19 (11): 767-76 (1987)
Half-life in blood /of rats/ 1.0 to 2.4 days... Half-life in liver /of rats/ 170-318 days /From table/.
California Department of Pesticide Regulation; Second Generation Anticoagulant Rodenticide Assessment, June 27, 2013; Available from, as of March 13, 2014: https://www.cdpr.ca.gov/docs/registration/reevaluation/chemicals/brodifacoum_final_assess.pdf
Calculated elimination half-life of 140 hr.
PMID:16803669 Grobosch T et al; J Anal Toxicol. 30(4):281-6 (2006).
The concentrations of bromadiolone in whole blood and plasma in serial samples from a 62-year-old woman were measured. The half-life of bromadiolone in blood was estimated to be about 6 days in the initial phase of elimination and about 10-13 days in the terminal phase.
PMID:18482378 Vindenes V; J Forensic Sci. 53(4):993-6 (2008).
An anticoagulant inhibiting the formation of prothrombin.
Spencer, E. Y. Guide to the Chemicals Used in Crop Protection. 7th ed. Publication 1093. Research Institute, Agriculture Canada, Ottawa, Canada: Information Canada, 1982., p. 52
Anticoagulant pesticides are used widely in agricultural and urban rodent control. The emergence of warfarin-resistant strains of rats led to the introduction of a new group of anticoagulant rodenticides variously referred to as 'superwarfarins', 'single dose' or 'long-acting'. This group includes the second generation 4-hydroxycoumarins brodifacoum, bromadiolone, difenacoum, flocoumafen and the indanedione derivatives chlorophacinone and diphacinone. Most cases of anticoagulant rodenticide exposure involve young children and, as a consequence, the amounts ingested are almost invariably small. In contrast, intentional ingestion of large quantities of long-acting anticoagulant rodenticides may cause anticoagulation for several weeks or months. Occupational exposure has also been reported. Anticoagulant rodenticides inhibit vitamin K(1)-2,3 epoxide reductase and thus the synthesis of vitamin K and subsequently clotting factors II, VII, IX and X. The greater potency and duration of action of long-acting anticoagulant rodenticides is attributed to their: (i) greater affinity for vitamin K(1)-2,3-epoxide reductase; (ii) ability to disrupt the vitamin K(1)-epoxide cycle at more than one point; (iii) hepatic accumulation; and (iv) unusually long biological half-lives due to high lipid solubility and enterohepatic circulation...
PMID:16499407 Watt BE et al; Toxicol Rev. 24(4):259-69 (2005).
The effects of coumarins and indandiones on prothrombin synthesis and conversion of vitamin k1 2,3-epoxide to vitamin k1 were measured. Results provided evidence for the proposed mechanism of action by preventing regeneration of vitamin k1 from its metabolite. /Coumarins and indandiones/
PMID:864593 REN P ET AL; J PHARMACOL EXP THER 201 (3): 541-6 (1977)
Both 4-hydroxycoumarin derivatives and indandiones (also known as oral anticoagulants) are antagonists of vitamin K. Their use as rodenticides is based on the inhibition of the vitamin K-dependent step in the synthesis of a number of blood coagulation factors. The vitamin K-dependent proteins ...in the coagulation cascade... are the procoagulant factors II (prothrombin), VII (proconvertin), IX (Christmas factor) and X (Stuart-Prower factor), and the coagulation-inhibiting proteins C and S. All these proteins are synthesized in the liver. Before they are released into the circulation the various precursor proteins undergo substantial (intracellular) post-translational modification. Vitamin K functions as a co-enzyme in one of these modifications, namely the carboxylation at well-defined positions of 10-12 glutamate residues into gamma-carboxyglutamate (Gla). The presence of these Gla residues is essential for the procoagulant activity of the various coagulations factors. Vitamin K hydroquinone (KH2) is the active co-enzyme, and its oxidation to vitamin K 2,3-epoxide (KO) provides the energy required for the carboxylation reaction. The epoxide is than recycled in two reduction steps mediated by the enzyme KO reductase... . The latter enzyme is the target enzyme for coumarin anticoagulants. Their blocking of the KO reductase leads to a rapid exhaustion of the supply of KH2, and thus to an effective prevention of the formation of Gla residues. This leads to an accumulation of non-carboxylated coagulation factor precursors in the liver. In some cases these precursors are processed further without being carboxylated, and (depending on the species) may appear in the circulation. At that stage the under-carboxylated proteins are designated as descarboxy coagulation factors. Normal coagulation factors circulate in the form of zymogens, which can only participate in the coagulation cascade after being activated by limited proteolytic degradation. Descarboxy coagulation factors have no procoagulant activity (i.e. they cannot be activated) and neither they can be converted into the active zymogens by vitamin K action. Whereas in anticoagulated humans high levels of circulating descarboxy coagulation factors are detectable, these levels are negligible in warfarin-treated rats and mice. /Anticoagulant rodenticides/
WHO; Environ Health Criteria 175: Anticoagulant Rodenticides p.46 (1995)
For more Mechanism of Action (Complete) data for BROMADIOLONE (6 total), please visit the HSDB record page.


