



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
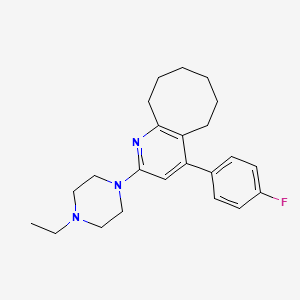

1. 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta(b)pyridine
2. Ad 5423
3. Ad-5423
4. Ad5423
5. Cycloocta(b)pyridine, 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-
6. Lonasen
1. 132810-10-7
2. Lonasen
3. Ad-5423
4. Blonanserin [inn]
5. Ad 5423
6. 2-(4-ethylpiperazin-1-yl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridine
7. Blonanserin (lonasen)
8. 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta(b)pyridine
9. Aq316b4f8c
10. Chembl178803
11. 132810-10-7 (free Base)
12. 2-(4-ethyl-1-piperazinyl)-4-(p-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta(b)pyridine
13. Ncgc00183858-01
14. 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridine
15. Cycloocta(b)pyridine, 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-
16. 2-(4-ethylpiperazin-1-yl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta[b]pyridine.
17. Blonanserin (jan/inn)
18. Unii-aq316b4f8c
19. 2-(4-ethylpiperazin-1-yl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydrocycloocta(b)pyridine
20. Cycloocta[b]pyridine, 2-(4-ethyl-1-piperazinyl)-4-(4-fluorophenyl)-5,6,7,8,9,10-hexahydro-
21. Ad5423
22. Blonanserin [mi]
23. Blonanserin [jan]
24. Blonanserin(ad-5423)
25. Blonanserin [mart.]
26. Dsstox_cid_28716
27. Dsstox_rid_82985
28. Dsstox_gsid_48790
29. Blonanserin [who-dd]
30. Schembl119669
31. Gtpl7670
32. Dtxsid7048790
33. Blonanserin, >=98% (hplc)
34. Chebi:31296
35. Amy8830
36. Ex-a663
37. Hms3740e13
38. Zinc597434
39. Bcp04236
40. Dsp-5423
41. Tox21_113267
42. Bdbm50160807
43. Mfcd00893838
44. Pdsp1_000720
45. Pdsp2_000710
46. S2112
47. Akos005145823
48. Ac-1599
49. Db09223
50. Sb17396
51. Cycloocta(b)pyridine, 2-(4-ethyl-1-piperazinyl)-4-(1-fluorophenyl)-5,6,7,8,9,10-hexahydro-
52. As-11421
53. Hy-13575
54. A8384
55. B4565
56. Cas-132810-10-7
57. Ft-0663415
58. Ad-5423;ad5423;ad 5423
59. D01176
60. Ab01565798_02
61. 810b107
62. L001392
63. Sr-01000945256
64. Q4927426
65. Sr-01000945256-1
66. 4-(4-fluoro-phenyl)-2-(4-propyl-piperazin-1-yl)-5,6,7,8,9,10-hexahydro-cycloocta[b]pyridine
| Molecular Weight | 367.5 g/mol |
|---|---|
| Molecular Formula | C23H30FN3 |
| XLogP3 | 5.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 367.24237613 g/mol |
| Monoisotopic Mass | 367.24237613 g/mol |
| Topological Polar Surface Area | 19.4 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 443 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used for the treatment of schizophrenia.
Blonanserin antagonizes dopamine and serotonin receptors to reduce symptoms of schizophrenia.
Absorption
Blonanserin has a Tmax of 1.5 h and a bioavailablity of 55%. Tmax has been observed to be prolonged and relative bioavailability increased when administered with food.
Route of Elimination
57% of blonanserin is excreted in the urine and 30% in the feces. Only 5% of the drug in the feces is the parent drug with no parent drug excreted through the urine.
Volume of Distribution
Blonanserin has a Vc of 9500 L and a Vt of 8560 L for a total Vd of 18060 L.
Clearance
Blonanserin has a clearance of 1230 L/h.
Blonanserin is mainly metabolized by CYP3A4. It undergoes hydoxylation of the cyclooctane ring as well as N-oxidation and N-deethylation of the piperazine ring. The N-deethylated and hydroxylated metabolites are active but to a lesser degree than the parent drug.
Blonanserin has a half life of elimination of 10.7-16.2 h.
Blonanserin binds to and inhibits dopamine receptors D2 and D3 as well as the serotonin receptor 5-HT2A with high affinity. Blonanserin has low affinity for other dopamine and serotonin receptors as well as muscarinic, adrenergic, and histamine receptors. This reduces dopaminergic and serotonergic neurotransmission which is thought to produce a reduction in positive and negative symptoms of schizophrenia respectively.


