


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
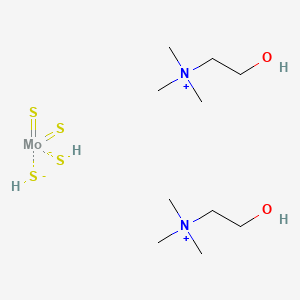

1. Ammonium Tetrathiomolybdate
2. Atn-224
3. Tetrathiomolybdate
4. Tetrathiomolybdate Bis-choline Salt
5. Tetrathiomolybdate, Dipotassium Salt
6. Tetrathiomolybdate, Disodium Salt
7. Thiomolybdate
8. Ttm Cpd
1. Atn-224
2. 649749-10-0
3. Atn 224
4. Bis-choline Tetrathiomolybdate
5. Tiomolibdate Choline
6. Tiomolibdate Choline [usan]
7. Alxn1840
8. Fd57a79r4p
9. Wtx-101
10. 2-hydroxy-n,n,n-trimethylethan-1-aminium Tetrathiomolybdate
11. Unii-fd57a79r4p
12. Choline Tetrathiomolybdate
13. Schembl3785098
14. Wtx101
15. Alxn-1840
16. Tiomolibdate Choline [jan]
17. Hy-16074
18. Bis(choline) Tetrathiomolybdate [who-dd]
19. Q17005864
20. 2-hydroxy-n,n,n-trimethylethanaminium Tetrathiomolybdate
21. Ethanaminium, 2-hydroxy-n,n,n-trimethyl-, (t-4)-tetrathioxomolybdate(2-) (2:1)
22. Ethanaminium, 2-hydroxy-n,n,n-trimethyl-, (t-4)-tetrathioxomolybdate(2-)(2:1)
| Molecular Weight | 434.6 g/mol |
|---|---|
| Molecular Formula | C10H30MoN2O2S4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 436.024417 g/mol |
| Monoisotopic Mass | 436.024417 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 65.7 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 5 |
Treatment of Wilson Disease
Angiogenesis Inhibitors
Agents and endogenous substances that antagonize or inhibit the development of new blood vessels. (See all compounds classified as Angiogenesis Inhibitors.)
Chelating Agents
Chemicals that bind to and remove ions from solutions. Many chelating agents function through the formation of COORDINATION COMPLEXES with METALS. (See all compounds classified as Chelating Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)


