
Synopsis
Synopsis
0
CEP/COS
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. 192024, Agn
2. Agn 192024
3. Latisse
4. Lumigan
1. 155206-00-1
2. Lumigan
3. Latisse
4. Agn 192024
5. Prostamide
6. Agn-192024
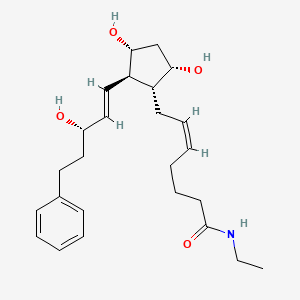
7. (z)-7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(e,3s)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-n-ethylhept-5-enamide
8. Qxs94885mz
9. (z)-7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((1e,3s)-3-hydroxy-5-phenyl-1-pentenyl)cyclopentyl)-n-ethyl-5-heptenamide
10. Chebi:51230
11. (e)-7-[3,5-dihydroxy-2-[(e)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-n-ethylhept-5-enamide
12. Bimatoprostum
13. Unii-qxs94885mz
14. (5z)-7-{(1r,2r,3r,5s)-3,5-dihydroxy-2-[(1e,3s)-3-hydroxy-5-phenylpent-1-en-1-yl]cyclopentyl}-n-ethylhept-5-enamide
15. (5z)-7-{(1r,2r,3r,5s)-3,5-dihydroxy-2-[(1e,3s)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl}-n-ethylhept-5-enamide
16. Lumigan (tn)
17. (z)-7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((s,e)-3-hydroxy-5-phenylpent-1-en-1-yl)cyclopentyl)-n-ethylhept-5-enamide
18. (5z)-7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(1e,3s)-3-hydroxy-5-phenylpent-1-en-1-yl]cyclopentyl]-n-ethylhept-5-enamide
19. Bimatoprost [usan:inn:ban:jan]
20. Bimatoprost In Bulk
21. Latisse (tn)
22. Durysta
23. Ls-181817
24. Bimatoprost [mi]
25. Bimatoprost [inn]
26. Bimatoprost [jan]
27. (5z)-bimatoprost
28. Bimatoprost [inci]
29. Bimatoprost [usan]
30. Bimatoprost [vandf]
31. Bimatoprost [mart.]
32. Schembl24425
33. Bimatoprost [who-dd]
34. 5-heptenamide, 7-(3,5-dihydroxy-2-(3-hydrdoxy-5-phenyl-1-pentenyl)cyclopentyl)-n-ethyl-, (1r-(1alpha(z),2beta(1e,3s*),3alpha,5alpha))-
35. Mls006010039
36. Us9271961, Bimatoprost
37. Bimatoprost (jan/usan/inn)
38. Bimatoprost [ema Epar]
39. Gtpl1958
40. Chembl1200963
41. Bimatoprost [orange Book]
42. Dtxsid30895042
43. Bdbm220120
44. Ex-a1769
45. Ganfort Component Bimatoprost
46. Hy-b0191
47. Zinc4474405
48. Mfcd03411999
49. Akos015995566
50. Am84507
51. Bimatoprost Component Of Ganfort
52. Db00905
53. Fd10460
54. Ncgc00181745-01
55. Ncgc00181745-03
56. 5-heptenamide, 7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((1e,3s)-3-hydroxy-5-phenyl-1-pentenyl)cyclopentyl)-n-ethyl-, (5z)-
57. 5-heptenamide, 7-(3,5-dihydroxy-2-(3-hydroxy-5-phenyl-1-pentenyl)cyclopentyl)-n-ethyl-, (1r-1(alpha(z),2beta(1e,3s*)3alpha,5alpha))-
58. As-35082
59. Smr000058996
60. B6165
61. D02724
62. 206b001
63. Sr-01000942224
64. Q2393348
65. Sr-01000942224-1
66. 17-phenyl Trinor Prostaglandin F2alpha Ethyl Amide
67. 17-phenyl-tri-norprostaglandin F2alpha-ethyl Amide, >=95%, Solid
68. 15m
69. 5-heptenamide, 7-(3,5-dihydroxy-2-(3-hydroxy-5-phenyl-1-pentenyl)cyclopentyl)-n-ethyl-, (1r-1(.alpha.(z),2.beta.(1e,3s*)3.alpha.,5.alpha.))-
70. 5-heptenamide, 7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(1e,3s)-3-hydroxy-5-phenyl-1-penten-1-yl]cyclopentyl]-n-ethyl-, (5z)-
| Molecular Weight | 415.6 g/mol |
|---|---|
| Molecular Formula | C25H37NO4 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 12 |
| Exact Mass | 415.27225866 g/mol |
| Monoisotopic Mass | 415.27225866 g/mol |
| Topological Polar Surface Area | 89.8 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 541 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Bimatoprost |
| PubMed Health | Bimatoprost (Into the eye) |
| Drug Classes | Antiglaucoma, Ophthalmologic Agent |
| Drug Label | LATISSE (bimatoprost ophthalmic solution) 0.03% is a synthetic prostaglandin analog. Its chemical name is (Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-N-ethyl-5-heptenamide, and its molecular weight is 41... |
| Active Ingredient | Bimatoprost |
| Dosage Form | Solution |
| Route | ophthalmic |
| Strength | 0.01%; 0.03%; 0.03 |
| Market Status | Tentative Approval |
| Company | Apotex; Sandoz |
| 2 of 6 | |
|---|---|
| Drug Name | Latisse |
| PubMed Health | Bimatoprost (Into the eye) |
| Drug Classes | Antiglaucoma, Ophthalmologic Agent |
| Drug Label | LATISSE (bimatoprost ophthalmic solution) 0.03% is a synthetic prostaglandin analog. Its chemical name is (Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-N-ethyl-5-heptenamide, and its molecular weight is 41... |
| Active Ingredient | Bimatoprost |
| Dosage Form | Solution/drops |
| Route | Topical |
| Strength | 0.03% |
| Market Status | Prescription |
| Company | Allergan |
| 3 of 6 | |
|---|---|
| Drug Name | Lumigan |
| Drug Label | LUMIGAN 0.01% and 0.03% (bimatoprost ophthalmic solution) is a synthetic prostamide analog with ocular hypotensive activity. Its chemical name is (Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-5-N-ethylhept... |
| Active Ingredient | Bimatoprost |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.01% |
| Market Status | Prescription |
| Company | Allergan |
| 4 of 6 | |
|---|---|
| Drug Name | Bimatoprost |
| PubMed Health | Bimatoprost (Into the eye) |
| Drug Classes | Antiglaucoma, Ophthalmologic Agent |
| Drug Label | LATISSE (bimatoprost ophthalmic solution) 0.03% is a synthetic prostaglandin analog. Its chemical name is (Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-N-ethyl-5-heptenamide, and its molecular weight is 41... |
| Active Ingredient | Bimatoprost |
| Dosage Form | Solution |
| Route | ophthalmic |
| Strength | 0.01%; 0.03%; 0.03 |
| Market Status | Tentative Approval |
| Company | Apotex; Sandoz |
| 5 of 6 | |
|---|---|
| Drug Name | Latisse |
| PubMed Health | Bimatoprost (Into the eye) |
| Drug Classes | Antiglaucoma, Ophthalmologic Agent |
| Drug Label | LATISSE (bimatoprost ophthalmic solution) 0.03% is a synthetic prostaglandin analog. Its chemical name is (Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-N-ethyl-5-heptenamide, and its molecular weight is 41... |
| Active Ingredient | Bimatoprost |
| Dosage Form | Solution/drops |
| Route | Topical |
| Strength | 0.03% |
| Market Status | Prescription |
| Company | Allergan |
| 6 of 6 | |
|---|---|
| Drug Name | Lumigan |
| Drug Label | LUMIGAN 0.01% and 0.03% (bimatoprost ophthalmic solution) is a synthetic prostamide analog with ocular hypotensive activity. Its chemical name is (Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-5-N-ethylhept... |
| Active Ingredient | Bimatoprost |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.01% |
| Market Status | Prescription |
| Company | Allergan |
Bimatoprost is used for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. These patients must be intolerant to other intraocular pressure lowering medications or inadequately responsive to other treatments. Bimatoprost is also indicated to treat eyelash hypotrichosis.
Reduction of elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension (as monotherapy or as adjunctive therapy to beta-blockers).
Treatment of glaucoma, Treatment of non-scarring hair loss
Treatment of androgenic alopecia
High intraocular pressure is a major risk factor for glaucoma-related visual field loss. A linear relationship exists between intraocular pressure and the risk of damaging the optic nerve, which can lead to considerable visual impairment. Therefore, conditions such as ocular hypertension and glaucoma can cause dangerous elevations of intraocular pressure. Bimatoprost rapidly decreases intraocular pressure and reduces the risk for visual field loss from ocular hypertension due to various causes. Other effects of this drug may include gradual changes in eyelid pigmentation, changes in iris pigmentation, changes in eyelash pigmentation, growth and thickness. Patients should be informed of these possible effects, especially if this drug is only administered to one eye, which may noticeably change in appearance with bimatoprost treatment.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
S01EE03
S - Sensory organs
S01 - Ophthalmologicals
S01E - Antiglaucoma preparations and miotics
S01EE - Prostaglandin analogues
S01EE03 - Bimatoprost
Absorption
This drug is absorbed systemically when administered to the eye. A study was performed on 15 healthy volunteers and bimatoprost ophthalmic solution 0.03% was administered once daily for 14 days. The mean Cmax was approximately 0.08 ng/mL and AUC0-24hr was approximately 0.09 on days 7 and 14 of the study. By 10 minutes, peak blood concentration was achieved. Bimatoprost was not detectable at 1.5 hours after administration in most subjects. The maximum blood concentration in a study of 6 healthy volunteers was determined to be 12.2 ng/mL. Steady state was reached in the first week of dosing. One drug label mentions that onset of decreased intraocular pressure occurs approximately 4 hours after the first administration and the peak effect occurs in the range of 8-12 hours. Bimatoprost effects may last up to 24 hours.
Route of Elimination
One pharmacokinetic study of bimatoprost in 6 healthy volunteers determined that 67% of the administered dose was found to be excreted in the urine while 25% of the dose was recovered in the feces.
Volume of Distribution
The volume of distribution at steady state is 0.67 L/kg.. It penetrates the human cornea and sclera.
Clearance
The clearance was measured to be 1.5 L/hr/kg in healthy subjects receiving IV administration of bimatoprost dosed at 3.12 ug/kg.
Bimatoprost is hydrolyzed to its active form, bimatoprost acid, in the eye. Bimatoprost undergoes oxidation, N-deethylation, and glucuronidation after it is systemically absorbed, and this leads to the production of various metabolites. In vitro studies show that CYP3A4 is an enzyme that participates in the metabolism of bimatoprost. Despite this, many enzymes and pathways metabolize bimatoprost, therefore, no significant drug-drug interactions are likely to occur. Glucuronidated metabolites comprise most of the excreted drug product in the blood, urine, and feces in rats.
The elimination half-life of bimatoprost is approximately 45 minutes.
Bimatoprost imitates the effects of prostamides, specifically prostaglandin F2. Bimatoprost mildly stimulates aqueous humor outflow, relieving elevated intraocular pressure and decreasing the risk of optic nerve damage. It is thought that bimatoprost reduces intraocular pressure (IOP) in humans by causing an increase in outflow of the aqueous humor via the trabecular meshwork and uveoscleral pathways. It achieves the above effects by decreasing tonographic resistance to aqueous humor outflow. Bimatoprost does not affect aqueous humor production.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
71
PharmaCompass offers a list of Bimatoprost API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Bimatoprost manufacturer or Bimatoprost supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Bimatoprost manufacturer or Bimatoprost supplier.
PharmaCompass also assists you with knowing the Bimatoprost API Price utilized in the formulation of products. Bimatoprost API Price is not always fixed or binding as the Bimatoprost Price is obtained through a variety of data sources. The Bimatoprost Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A bimatoprostum manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of bimatoprostum, including repackagers and relabelers. The FDA regulates bimatoprostum manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. bimatoprostum API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of bimatoprostum manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A bimatoprostum supplier is an individual or a company that provides bimatoprostum active pharmaceutical ingredient (API) or bimatoprostum finished formulations upon request. The bimatoprostum suppliers may include bimatoprostum API manufacturers, exporters, distributors and traders.
click here to find a list of bimatoprostum suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A bimatoprostum DMF (Drug Master File) is a document detailing the whole manufacturing process of bimatoprostum active pharmaceutical ingredient (API) in detail. Different forms of bimatoprostum DMFs exist exist since differing nations have different regulations, such as bimatoprostum USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A bimatoprostum DMF submitted to regulatory agencies in the US is known as a USDMF. bimatoprostum USDMF includes data on bimatoprostum's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The bimatoprostum USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of bimatoprostum suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The bimatoprostum Drug Master File in Japan (bimatoprostum JDMF) empowers bimatoprostum API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the bimatoprostum JDMF during the approval evaluation for pharmaceutical products. At the time of bimatoprostum JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of bimatoprostum suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a bimatoprostum Drug Master File in Korea (bimatoprostum KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of bimatoprostum. The MFDS reviews the bimatoprostum KDMF as part of the drug registration process and uses the information provided in the bimatoprostum KDMF to evaluate the safety and efficacy of the drug.
After submitting a bimatoprostum KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their bimatoprostum API can apply through the Korea Drug Master File (KDMF).
click here to find a list of bimatoprostum suppliers with KDMF on PharmaCompass.
A bimatoprostum written confirmation (bimatoprostum WC) is an official document issued by a regulatory agency to a bimatoprostum manufacturer, verifying that the manufacturing facility of a bimatoprostum active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting bimatoprostum APIs or bimatoprostum finished pharmaceutical products to another nation, regulatory agencies frequently require a bimatoprostum WC (written confirmation) as part of the regulatory process.
click here to find a list of bimatoprostum suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing bimatoprostum as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for bimatoprostum API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture bimatoprostum as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain bimatoprostum and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a bimatoprostum NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of bimatoprostum suppliers with NDC on PharmaCompass.
bimatoprostum Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of bimatoprostum GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right bimatoprostum GMP manufacturer or bimatoprostum GMP API supplier for your needs.
A bimatoprostum CoA (Certificate of Analysis) is a formal document that attests to bimatoprostum's compliance with bimatoprostum specifications and serves as a tool for batch-level quality control.
bimatoprostum CoA mostly includes findings from lab analyses of a specific batch. For each bimatoprostum CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
bimatoprostum may be tested according to a variety of international standards, such as European Pharmacopoeia (bimatoprostum EP), bimatoprostum JP (Japanese Pharmacopeia) and the US Pharmacopoeia (bimatoprostum USP).

