



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports

1. 192024, Agn
2. Agn 192024
3. Latisse
4. Lumigan
1. 155206-00-1
2. Lumigan
3. Latisse
4. Agn 192024
5. Prostamide
6. Agn-192024
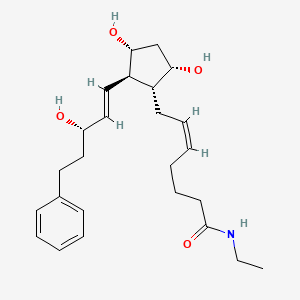
7. (z)-7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(e,3s)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-n-ethylhept-5-enamide
8. Qxs94885mz
9. (z)-7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((1e,3s)-3-hydroxy-5-phenyl-1-pentenyl)cyclopentyl)-n-ethyl-5-heptenamide
10. Chebi:51230
11. (e)-7-[3,5-dihydroxy-2-[(e)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl]-n-ethylhept-5-enamide
12. Bimatoprostum
13. Unii-qxs94885mz
14. (5z)-7-{(1r,2r,3r,5s)-3,5-dihydroxy-2-[(1e,3s)-3-hydroxy-5-phenylpent-1-en-1-yl]cyclopentyl}-n-ethylhept-5-enamide
15. (5z)-7-{(1r,2r,3r,5s)-3,5-dihydroxy-2-[(1e,3s)-3-hydroxy-5-phenylpent-1-enyl]cyclopentyl}-n-ethylhept-5-enamide
16. Lumigan (tn)
17. (z)-7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((s,e)-3-hydroxy-5-phenylpent-1-en-1-yl)cyclopentyl)-n-ethylhept-5-enamide
18. (5z)-7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(1e,3s)-3-hydroxy-5-phenylpent-1-en-1-yl]cyclopentyl]-n-ethylhept-5-enamide
19. Bimatoprost [usan:inn:ban:jan]
20. Bimatoprost In Bulk
21. Latisse (tn)
22. Durysta
23. Ls-181817
24. Bimatoprost [mi]
25. Bimatoprost [inn]
26. Bimatoprost [jan]
27. (5z)-bimatoprost
28. Bimatoprost [inci]
29. Bimatoprost [usan]
30. Bimatoprost [vandf]
31. Bimatoprost [mart.]
32. Schembl24425
33. Bimatoprost [who-dd]
34. 5-heptenamide, 7-(3,5-dihydroxy-2-(3-hydrdoxy-5-phenyl-1-pentenyl)cyclopentyl)-n-ethyl-, (1r-(1alpha(z),2beta(1e,3s*),3alpha,5alpha))-
35. Mls006010039
36. Us9271961, Bimatoprost
37. Bimatoprost (jan/usan/inn)
38. Bimatoprost [ema Epar]
39. Gtpl1958
40. Chembl1200963
41. Bimatoprost [orange Book]
42. Dtxsid30895042
43. Bdbm220120
44. Ex-a1769
45. Ganfort Component Bimatoprost
46. Hy-b0191
47. Zinc4474405
48. Mfcd03411999
49. Akos015995566
50. Am84507
51. Bimatoprost Component Of Ganfort
52. Db00905
53. Fd10460
54. Ncgc00181745-01
55. Ncgc00181745-03
56. 5-heptenamide, 7-((1r,2r,3r,5s)-3,5-dihydroxy-2-((1e,3s)-3-hydroxy-5-phenyl-1-pentenyl)cyclopentyl)-n-ethyl-, (5z)-
57. 5-heptenamide, 7-(3,5-dihydroxy-2-(3-hydroxy-5-phenyl-1-pentenyl)cyclopentyl)-n-ethyl-, (1r-1(alpha(z),2beta(1e,3s*)3alpha,5alpha))-
58. As-35082
59. Smr000058996
60. B6165
61. D02724
62. 206b001
63. Sr-01000942224
64. Q2393348
65. Sr-01000942224-1
66. 17-phenyl Trinor Prostaglandin F2alpha Ethyl Amide
67. 17-phenyl-tri-norprostaglandin F2alpha-ethyl Amide, >=95%, Solid
68. 15m
69. 5-heptenamide, 7-(3,5-dihydroxy-2-(3-hydroxy-5-phenyl-1-pentenyl)cyclopentyl)-n-ethyl-, (1r-1(.alpha.(z),2.beta.(1e,3s*)3.alpha.,5.alpha.))-
70. 5-heptenamide, 7-[(1r,2r,3r,5s)-3,5-dihydroxy-2-[(1e,3s)-3-hydroxy-5-phenyl-1-penten-1-yl]cyclopentyl]-n-ethyl-, (5z)-
| Molecular Weight | 415.6 g/mol |
|---|---|
| Molecular Formula | C25H37NO4 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 12 |
| Exact Mass | 415.27225866 g/mol |
| Monoisotopic Mass | 415.27225866 g/mol |
| Topological Polar Surface Area | 89.8 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 541 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Bimatoprost |
| PubMed Health | Bimatoprost (Into the eye) |
| Drug Classes | Antiglaucoma, Ophthalmologic Agent |
| Drug Label | LATISSE (bimatoprost ophthalmic solution) 0.03% is a synthetic prostaglandin analog. Its chemical name is (Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-N-ethyl-5-heptenamide, and its molecular weight is 41... |
| Active Ingredient | Bimatoprost |
| Dosage Form | Solution |
| Route | ophthalmic |
| Strength | 0.01%; 0.03%; 0.03 |
| Market Status | Tentative Approval |
| Company | Apotex; Sandoz |
| 2 of 6 | |
|---|---|
| Drug Name | Latisse |
| PubMed Health | Bimatoprost (Into the eye) |
| Drug Classes | Antiglaucoma, Ophthalmologic Agent |
| Drug Label | LATISSE (bimatoprost ophthalmic solution) 0.03% is a synthetic prostaglandin analog. Its chemical name is (Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-N-ethyl-5-heptenamide, and its molecular weight is 41... |
| Active Ingredient | Bimatoprost |
| Dosage Form | Solution/drops |
| Route | Topical |
| Strength | 0.03% |
| Market Status | Prescription |
| Company | Allergan |
| 3 of 6 | |
|---|---|
| Drug Name | Lumigan |
| Drug Label | LUMIGAN 0.01% and 0.03% (bimatoprost ophthalmic solution) is a synthetic prostamide analog with ocular hypotensive activity. Its chemical name is (Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-5-N-ethylhept... |
| Active Ingredient | Bimatoprost |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.01% |
| Market Status | Prescription |
| Company | Allergan |
| 4 of 6 | |
|---|---|
| Drug Name | Bimatoprost |
| PubMed Health | Bimatoprost (Into the eye) |
| Drug Classes | Antiglaucoma, Ophthalmologic Agent |
| Drug Label | LATISSE (bimatoprost ophthalmic solution) 0.03% is a synthetic prostaglandin analog. Its chemical name is (Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-N-ethyl-5-heptenamide, and its molecular weight is 41... |
| Active Ingredient | Bimatoprost |
| Dosage Form | Solution |
| Route | ophthalmic |
| Strength | 0.01%; 0.03%; 0.03 |
| Market Status | Tentative Approval |
| Company | Apotex; Sandoz |
| 5 of 6 | |
|---|---|
| Drug Name | Latisse |
| PubMed Health | Bimatoprost (Into the eye) |
| Drug Classes | Antiglaucoma, Ophthalmologic Agent |
| Drug Label | LATISSE (bimatoprost ophthalmic solution) 0.03% is a synthetic prostaglandin analog. Its chemical name is (Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-N-ethyl-5-heptenamide, and its molecular weight is 41... |
| Active Ingredient | Bimatoprost |
| Dosage Form | Solution/drops |
| Route | Topical |
| Strength | 0.03% |
| Market Status | Prescription |
| Company | Allergan |
| 6 of 6 | |
|---|---|
| Drug Name | Lumigan |
| Drug Label | LUMIGAN 0.01% and 0.03% (bimatoprost ophthalmic solution) is a synthetic prostamide analog with ocular hypotensive activity. Its chemical name is (Z)-7-[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-5-N-ethylhept... |
| Active Ingredient | Bimatoprost |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.01% |
| Market Status | Prescription |
| Company | Allergan |
Bimatoprost is used for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. These patients must be intolerant to other intraocular pressure lowering medications or inadequately responsive to other treatments. Bimatoprost is also indicated to treat eyelash hypotrichosis.
Reduction of elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension (as monotherapy or as adjunctive therapy to beta-blockers).
Treatment of glaucoma, Treatment of non-scarring hair loss
Treatment of androgenic alopecia
High intraocular pressure is a major risk factor for glaucoma-related visual field loss. A linear relationship exists between intraocular pressure and the risk of damaging the optic nerve, which can lead to considerable visual impairment. Therefore, conditions such as ocular hypertension and glaucoma can cause dangerous elevations of intraocular pressure. Bimatoprost rapidly decreases intraocular pressure and reduces the risk for visual field loss from ocular hypertension due to various causes. Other effects of this drug may include gradual changes in eyelid pigmentation, changes in iris pigmentation, changes in eyelash pigmentation, growth and thickness. Patients should be informed of these possible effects, especially if this drug is only administered to one eye, which may noticeably change in appearance with bimatoprost treatment.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
S01EE03
S - Sensory organs
S01 - Ophthalmologicals
S01E - Antiglaucoma preparations and miotics
S01EE - Prostaglandin analogues
S01EE03 - Bimatoprost
Absorption
This drug is absorbed systemically when administered to the eye. A study was performed on 15 healthy volunteers and bimatoprost ophthalmic solution 0.03% was administered once daily for 14 days. The mean Cmax was approximately 0.08 ng/mL and AUC0-24hr was approximately 0.09 on days 7 and 14 of the study. By 10 minutes, peak blood concentration was achieved. Bimatoprost was not detectable at 1.5 hours after administration in most subjects. The maximum blood concentration in a study of 6 healthy volunteers was determined to be 12.2 ng/mL. Steady state was reached in the first week of dosing. One drug label mentions that onset of decreased intraocular pressure occurs approximately 4 hours after the first administration and the peak effect occurs in the range of 8-12 hours. Bimatoprost effects may last up to 24 hours.
Route of Elimination
One pharmacokinetic study of bimatoprost in 6 healthy volunteers determined that 67% of the administered dose was found to be excreted in the urine while 25% of the dose was recovered in the feces.
Volume of Distribution
The volume of distribution at steady state is 0.67 L/kg.. It penetrates the human cornea and sclera.
Clearance
The clearance was measured to be 1.5 L/hr/kg in healthy subjects receiving IV administration of bimatoprost dosed at 3.12 ug/kg.
Bimatoprost is hydrolyzed to its active form, bimatoprost acid, in the eye. Bimatoprost undergoes oxidation, N-deethylation, and glucuronidation after it is systemically absorbed, and this leads to the production of various metabolites. In vitro studies show that CYP3A4 is an enzyme that participates in the metabolism of bimatoprost. Despite this, many enzymes and pathways metabolize bimatoprost, therefore, no significant drug-drug interactions are likely to occur. Glucuronidated metabolites comprise most of the excreted drug product in the blood, urine, and feces in rats.
The elimination half-life of bimatoprost is approximately 45 minutes.
Bimatoprost imitates the effects of prostamides, specifically prostaglandin F2. Bimatoprost mildly stimulates aqueous humor outflow, relieving elevated intraocular pressure and decreasing the risk of optic nerve damage. It is thought that bimatoprost reduces intraocular pressure (IOP) in humans by causing an increase in outflow of the aqueous humor via the trabecular meshwork and uveoscleral pathways. It achieves the above effects by decreasing tonographic resistance to aqueous humor outflow. Bimatoprost does not affect aqueous humor production.


