



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
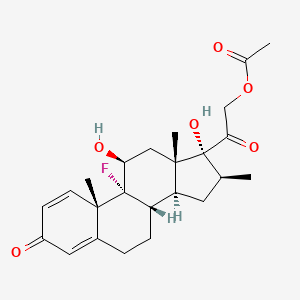

1. 987-24-6
2. Betamethasone 21-acetate
3. Beta-methasone Acetate
4. Ti05ao53l7
5. Chebi:31275
6. 9-fluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 21-acetate
7. Nsc-759196
8. (11b,16a)-9-fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione 21-acetate
9. (11beta,16beta)-9-fluoro-11,17-dihydroxy-16-methyl-3,20-dioxopregna-1,4-dien-21-yl Acetate
10. 9-fluoro-11beta,17-dihydroxy-16beta-methyl-3,20-dioxopregna-1,4-dien-21-yl Acetate
11. [2-[(8s,9r,10s,11s,13s,14s,16s,17r)-9-fluoro-11,17-dihydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Acetate
12. Betamethasone Acetate [jan]
13. Unii-ti05ao53l7
14. Betamethasone Acetate [usp:jan]
15. Betamethasone-acetate
16. Einecs 213-578-6
17. 9alpha-fluoro-11beta,17alpha,21-trihydroxy-16beta-methyl-1,4-pregnadiene-3,20-dione 21-acetate
18. Betamethason-21-acetat
19. Schembl7592
20. Betamethasone21-acetate
21. Chembl1200538
22. Dtxsid8022668
23. Betamethasone Acetate (jan/usp)
24. 9-fluoro-11.beta.,17,21-trihydroxy-16.beta.-methylpregna-1,4-diene-3,20-dione 21-acetate
25. Amy39051
26. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17-dihydroxy-16-methyl-21-(acetyloxy)-, (11.beta.,16.beta.)-
27. Zinc4212130
28. Betamethasone Acetate [vandf]
29. Betamethasone Acetate [mart.]
30. Akos015896575
31. Betamethasone Acetate [usp-rs]
32. Betamethasone Acetate [who-dd]
33. Nsc 759196
34. Betamethasone Acetate [green Book]
35. Betamethasone Acetate [ep Impurity]
36. Betamethasone Acetate [orange Book]
37. Betamethasone Acetate [ep Monograph]
38. B3165
39. Betamethasone Acetate [usp Monograph]
40. Osurnia Component Betamethasone Acetate
41. D01402
42. T71509
43. Betamethasone Acetate 100 Microg/ml In Methanol
44. Betamethasone Acetate Component Of Osurnia
45. 987m246
46. Betamethasone Valerate Impurity A [ep Impurity]
47. Dexamethasone Acetate Impurity D [ep Impurity]
48. Q27114254
49. Celestone Soluspan Component Betamethasone Acetate
50. Betamethasone Acetate Component Of Celestone Soluspan
51. Betamethasone Acetate, European Pharmacopoeia (ep) Reference Standard
52. Betamethasone Acetate, United States Pharmacopeia (usp) Reference Standard
53. Betamethasone Acetate, Pharmaceutical Secondary Standard; Certified Reference Material
54. 9alpha-fluoro-16beta-methyl-11beta,17alpha,21-trihydroxy-1,4-pregnadiene-3,20-dione 21-acetate
55. Pregna-1,4-diene-3,20-dione, 21-(acetyloxy)-9-fluoro-11,17-dihydroxy-16-methyl-, (11beta,16beta)-
56. Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17-dihydroxy-16-methyl-21-(acetyloxy)-, (11beta,16beta)-
| Molecular Weight | 434.5 g/mol |
|---|---|
| Molecular Formula | C24H31FO6 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 434.21046687 g/mol |
| Monoisotopic Mass | 434.21046687 g/mol |
| Topological Polar Surface Area | 101 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 910 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)






