



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
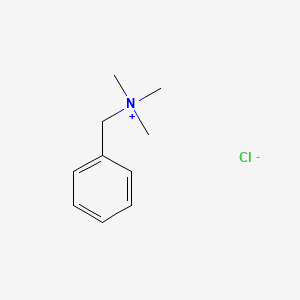

1. Benzyltrimethylammonium
2. Benzyltrimethylammonium Acetate
3. Benzyltrimethylammonium Bromide
4. Benzyltrimethylammonium Butanoate
5. Benzyltrimethylammonium Carbonate (2:1)
6. Benzyltrimethylammonium Formate
7. Benzyltrimethylammonium Heptanoate
8. Benzyltrimethylammonium Hexafluorophosphate (1-)
9. Benzyltrimethylammonium Hexanoate
10. Benzyltrimethylammonium Hydroxide
11. Benzyltrimethylammonium Iodide
12. Benzyltrimethylammonium Methoxide
13. Benzyltrimethylammonium Nonanoate
14. Benzyltrimethylammonium Octanoate
15. Benzyltrimethylammonium Pentanoate
16. Benzyltrimethylammonium Propanoate
1. 56-93-9
2. N,n,n-trimethyl-1-phenylmethanaminium Chloride
3. Benzyl Trimethyl Ammonium Chloride
4. Benzyl(trimethyl)azanium;chloride
5. Benzenemethanaminium, N,n,n-trimethyl-, Chloride
6. Benzyltrimethyl Ammonium Chloride
7. Trimethylbenzylammonium Chloride
8. Benzyl Trimethylammonium Chloride
9. Ammonium, Benzyltrimethyl-, Chloride
10. Vnk45y7ba1
11. Mls000069800
12. N,n,n-trimethyl(phenyl)methanaminium Chloride
13. Tmbac
14. Smr000059199
15. Dsstox_cid_4600
16. Dsstox_rid_77464
17. Dsstox_gsid_24600
18. Benzenemethanaminium, N,n,n-trimethyl-, Chloride (1:1)
19. Cas-56-93-9
20. Ccris 4587
21. Hsdb 4196
22. Einecs 200-300-3
23. Mfcd00011782
24. Unii-vnk45y7ba1
25. Benzyltrimethylazanium Chloride
26. N,n,n-trimethylbenzenemethanaminium Chloride
27. Trimethylbenzyl Ammonium Chloride
28. Hipochem Migrator J
29. Opera_id_418
30. Ec 200-300-3
31. Variquat B 200
32. Schembl2548
33. Benzyltrimethylamonium Chloride
34. Btmac 100
35. Benzyltrimethylarnmonium Chloride
36. Benzyl-trimethylammonium Chloride
37. Benzyltrimethyl-ammonium Chloride
38. Trimethyl-benzylammonium Chloride
39. Benzyl-trimethyl-azanium Chloride
40. Chembl1372143
41. Dtxsid8024600
42. Benzyl-trimethyl Ammonium Chloride
43. Benzyl-trimethyl-ammonium Chloride
44. N-benzyltrimethylammonium Chloride
45. Hms2232j08
46. Hms3371a05
47. Bcp24461
48. Tox21_201587
49. Tox21_303260
50. Akos001098667
51. Jc10026
52. Trimethyl-(phenylmethyl)azanium Chloride
53. Ncgc00090720-01
54. Ncgc00090720-02
55. Ncgc00257093-01
56. Ncgc00259136-01
57. Trimethyl-(phenylmethyl)ammonium Chloride
58. As-11734
59. Db-050364
60. B0447
61. Cs-0019417
62. Ft-0622785
63. N,n,n-trimethyl-1-phenylmethanaminiumchloride
64. Trimethylbenzylammonium Chloride [hsdb]
65. Benzyltrimethylammonium Chloride (x% In Water)
66. D72531
67. N-benzyl-n,n,n-trimethylammonium Chloride
68. A831246
69. W-105497
70. Q22829137
71. F8880-1007
72. Benzyltrimethylammonium Chloride Solution Technical, ~60% In H2o
73. Benzyltrimethylammonium Chloride, 60% Aqueous Solution
74. Benzyltrimethylammonium Chloride, 60%???aqueous Solution
| Molecular Weight | 185.69 g/mol |
|---|---|
| Molecular Formula | C10H16ClN |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 185.0971272 g/mol |
| Monoisotopic Mass | 185.0971272 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 107 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
Surface-Active Agents
Agents that modify interfacial tension of water; usually substances that have one lipophilic and one hydrophilic group in the molecule; includes soaps, detergents, emulsifiers, dispersing and wetting agents, and several groups of antiseptics. (See all compounds classified as Surface-Active Agents.)
IT DECR THE RESTING MEMBRANE POTENTIALS & BLOCKED ACTION POTENTIAL OF BOTH INNERVATED & DENERVATED MUSCLES. BTM MAY HAVE DUAL MECHANISM OF ACTION, A DEPOLARIZING TYPE & A BLOCKING ACTION WHICH IS DOSE DEPENDENT.
PMID:5087397 DRETCHEN ET AL; J PHARMACOL EXP THER 178 (1): 192 (1971)
ACETYLCHOLINE-LIKE AGONIST ACTIVITY ON CAT SUPERIOR CERVICAL GANGLION IN VIVO & FROG RECTUS ABDOMINIS IN VITRO WAS STUDIED. BENZYLTRIMETHYLAMMONIUM WAS MORE ACTIVE THAN ACETYLCHOLINE AS GANGLION STIMULANT.
PMID:5639098 HAMILTON JT, RUBINSTEIN HM; J PHARMACOL EXP THER 160 (1): 112 (1968)
IT WAS TESTED FOR MUSCARINIC ACTIVITY RELATIVE TO ACETYLCHOLINE DURING NONDEPOLARIZING BLOCKAGE DUE TO NICOTINE 1.2 MG/MIN IV & WAS FOUND TO BE MORE ACTIVE THAN ACETYLCHOLINE. IT IS CAPABLE OF STIMULATING BOTH NICOTINIC & MUSCARINIC RECEPTORS.
PMID:5639098 HAMILTON JT, RUBINSTEIN HM; J PHARMACOL EXP THER 160 (1): 112 (1968)


