



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada

Australia

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
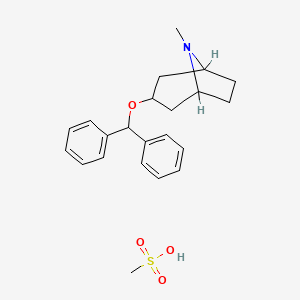

1. Apo Benztropine
2. Apo-benztropine
3. Bensylate
4. Benzatropine
5. Benzatropine Mesylate
6. Benzatropine Methanesulfonate
7. Benzatropine Methanesulfonate, Hydrobromide
8. Benzatropine Methanesulfonate, Hydrobromide, (endo)-isomer
9. Benzatropine Methanesulfonate, Hydrochloride, (endo)-isomer
10. Benztropine
11. Benztropine Mesylate
12. Cogentin
13. Cogentinol
14. Hydrobromide Benzatropine Methanesulfonate
15. Mesylate, Benzatropine
16. Mesylate, Benztropine
17. Methanesulfonate, Benzatropine
18. Methanesulfonate, Hydrobromide Benzatropine
19. N Methylbenztropine
20. N-methylbenztropine
21. Pms Benztropine
22. Pms-benztropine
1. Benztropine Mesylate
2. 132-17-2
3. Cogentin Mesylate
4. Benztropine (mesylate)
5. Mls000737056
6. Nsc42199
7. Benzotropine Mesylate
8. 3-(benzhydryloxy)-8-methyl-8-azabicyclo[3.2.1]octane Methanesulfonate
9. Smr000394012
10. Cobrentin Methanesulfonate
11. 3-benzhydryloxy-8-methyl-8-azabicyclo[3.2.1]octane;methanesulfonic Acid
12. Mk 02
13. Benzatropine Mesylate;benzotropine Mesylate;benztropine Methanesulfonate
14. Tropine Benzohydryl Ether Methanesulfonate
15. 102701-02-0
16. Cogentin Methanesulfonate
17. Sr-01000075303
18. Benzotropine Methanesulfonate
19. Nsc-42199
20. Benzotropinemesylate
21. Opera_id_1229
22. Schembl41809
23. Chembl85236
24. Hy-b0520a
25. Hms2747a15
26. Hms3259p11
27. Hms3260h09
28. Hms3652k07
29. Tox21_500194
30. Nsc169913
31. 3-(diphenylmethoxy)-8-methyl-8-azabicyclo[3.2.1]octane, Methanesulfonic Acid
32. Akos008105137
33. Ccg-221498
34. Lp00194
35. Nc00646
36. Sb19689
37. Ncgc00016118-02
38. Ncgc00093670-01
39. Ncgc00260879-01
40. As-17846
41. B5592
42. Cs-0300867
43. Eu-0100194
44. En300-51031
45. B 8262
46. Wln: T56 A Antj A1 Goyr & R & Osw1
47. Benzophenone-3,3'-4,4'-tetracarboxylic Dianhydrie
48. Q-200693
49. Sr-01000075303-1
50. Sr-01000075303-5
51. 8-azabicyclo[3.2.1]octane, Endo-, Methanesulfonate
52. 1.alpha.h, 3.alpha.-(diphenylmethoxy)-, Methanesulfonate
53. 3-(diphenylmethoxy)-8-methyl-8-azabicyclo[3.2.1]octane,methanesulfonicacid
54. Benzhydryl 8-methyl-8-azabicyclo[3.2.1]oct-3-yl Ether Methanesulfonate
| Molecular Weight | 403.5 g/mol |
|---|---|
| Molecular Formula | C22H29NO4S |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 403.18172958 g/mol |
| Monoisotopic Mass | 403.18172958 g/mol |
| Topological Polar Surface Area | 75.2 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 433 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Benztropine mesylate |
| PubMed Health | Benztropine Mesylate (By mouth) |
| Drug Classes | Antiparkinsonian |
| Drug Label | Benztropine Mesylate is a synthetic compound containing structural features found in atropine and diphenhydramine.It is a crystalline white powder, very soluble in water, designated as 3-(Diphenylmethoxy)-1H, 5H-tropane methanesulfonate, with t... |
| Active Ingredient | Benztropine mesylate |
| Dosage Form | Injectable; Tablet |
| Route | Injection; Oral |
| Strength | 1mg/ml; 0.5mg; 2mg; 1mg |
| Market Status | Prescription |
| Company | Corepharma; Navinta; Fresenius Kabi Usa; Excellium; Vintage; Usl Pharma; Luitpold; Hikma Farmaceutica; Pliva; Invagen Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Benztropine mesylate |
| PubMed Health | Benztropine Mesylate (By mouth) |
| Drug Classes | Antiparkinsonian |
| Drug Label | Benztropine Mesylate is a synthetic compound containing structural features found in atropine and diphenhydramine.It is a crystalline white powder, very soluble in water, designated as 3-(Diphenylmethoxy)-1H, 5H-tropane methanesulfonate, with t... |
| Active Ingredient | Benztropine mesylate |
| Dosage Form | Injectable; Tablet |
| Route | Injection; Oral |
| Strength | 1mg/ml; 0.5mg; 2mg; 1mg |
| Market Status | Prescription |
| Company | Corepharma; Navinta; Fresenius Kabi Usa; Excellium; Vintage; Usl Pharma; Luitpold; Hikma Farmaceutica; Pliva; Invagen Pharms |
Dopamine Uptake Inhibitors
Drugs that block the transport of DOPAMINE into axon terminals or into storage vesicles within terminals. Most of the ADRENERGIC UPTAKE INHIBITORS also inhibit dopamine uptake. (See all compounds classified as Dopamine Uptake Inhibitors.)
Antiparkinson Agents
Agents used in the treatment of Parkinson's disease. The most commonly used drugs act on the dopaminergic system in the striatum and basal ganglia or are centrally acting muscarinic antagonists. (See all compounds classified as Antiparkinson Agents.)
Parasympatholytics
Agents that inhibit the actions of the parasympathetic nervous system. The major group of drugs used therapeutically for this purpose is the MUSCARINIC ANTAGONISTS. (See all compounds classified as Parasympatholytics.)
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)






