



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
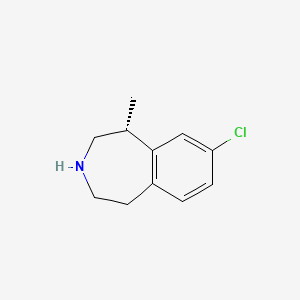

1. (1r)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1h-3-benzazepine
2. 8-chloro-2,3,4,5-tetrahydro-1-methyl-1h-3-benzazepine
3. Apd 356
4. Apd-356
5. Apd356
6. Ar-10a
7. Belviq
1. 616202-92-7
2. (r)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1h-benzo[d]azepine
3. Belviq
4. (1r)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1h-3-benzazepine
5. Lorcaserin [inn]
6. 1h-3-benzazepine, 8-chloro-2,3,4,5-tetrahydro-1-methyl-, (1r)-
7. (r)-8-chloro-1-methyl-2,3,4,5-tetrahydro-1h-3-benzazepine
8. Chembl360328
9. Chebi:65353
10. 637e494o0z
11. Ncgc00182550-01
12. Ar-10a
13. Dsstox_cid_28585
14. Dsstox_rid_82856
15. Dsstox_gsid_48659
16. Belvig
17. Cas-616202-92-7
18. Unii-637e494o0z
19. Hsdb 8128
20. T4u
21. Lorcaserin [mi]
22. (1r)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1h-3-benzazepine
23. Lorcaserin [vandf]
24. Lorcaserin [who-dd]
25. Schembl169382
26. Gtpl2941
27. Dtxsid3048659
28. 8-chloro-2,3,4,5-tetrahydro-1-methyl-1h-3-benzazepine
29. (5r)-7-chloro-5-methyl-2,3,4,5-tetrahydro-1h-3-benzazepine
30. Zinc6733300
31. Tox21_113018
32. Ac-553
33. Bdbm50161646
34. Akos006326204
35. Tox21_113018_1
36. Am81248
37. Cs-1232
38. Db04871
39. Ncgc00182550-02
40. Hy-11093
41. 202l927
42. Q340139
| Molecular Weight | 195.69 g/mol |
|---|---|
| Molecular Formula | C11H14ClN |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 195.0814771 g/mol |
| Monoisotopic Mass | 195.0814771 g/mol |
| Topological Polar Surface Area | 12 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 172 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | BELVIQ |
| Active Ingredient | LORCASERIN HYDROCHLORIDE |
| Company | EISAI INC (Application Number: N022529. Patents: 6953787, 7514422, 7977329, 8168624, 8207158, 8273734, 8367657, 8546379, 8575149, 8697686, 8946207, 8980881, 8999970, 9169213, 9770455) |
Belviq is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial body mass index (BMI) of: 30 kg/sq m or greater (obese), or 27 kg/sq m or greater (overweight) in the presence of at least one weight related comorbid condition (e.g., hypertension, dyslipidemia, type 2 diabetes). /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for BELVIQ (lorcaserin hydrochloride) tablets (August 2012). Available from, as of March 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cbbb12f-760d-487d-b789-ae2d52a3e01f
Belviq is a serotonergic drug. The development of a potentially life-threatening serotonin syndrome or Neuroleptic Malignant Syndrome (NMS)-like reactions have been reported during use of serotonergic drugs, including, but not limited to, selective serotonin-norepinephrine reuptake inhibitors (SNRIs) and selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), bupropion, triptans, dietary supplements such as St. John's Wort and tryptophan, drugs that impair metabolism of serotonin (including monoamine oxidase inhibitors (MAOIs)), dextromethorphan, lithium, tramadol, antipsychotics or other dopamine antagonists, particularly when used in combination. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Serotonin syndrome, in its most severe form, can resemble neuroleptic malignant syndrome, which includes hyperthermia, muscle rigidity, autonomic instability with possible rapid fluctuation of vital signs, and mental status changes. Patients should be monitored for the emergence of serotonin syndrome or NMS-like signs and symptoms.
US Natl Inst Health; DailyMed. Current Medication Information for BELVIQ (lorcaserin hydrochloride) tablets (August 2012). Available from, as of March 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cbbb12f-760d-487d-b789-ae2d52a3e01f
Regurgitant cardiac valvular disease, primarily affecting the mitral and/or aortic valves, has been reported in patients who took serotonergic drugs with 5-HT2B receptor agonist activity. The etiology of the regurgitant valvular disease is thought to be activation of 5-HT2B receptors on cardiac interstitial cells. At therapeutic concentrations, Belviq is selective for 5-HT2C receptors as compared to 5-HT2B receptors. In clinical trials of 1-year duration, 2.4% of patients receiving Belviq and 2.0% of patients receiving placebo developed echocardiographic criteria for valvular regurgitation at one year (mild or greater aortic regurgitation and/or moderate or greater mitral regurgitation): none of these patients was symptomatic.
US Natl Inst Health; DailyMed. Current Medication Information for BELVIQ (lorcaserin hydrochloride) tablets (August 2012). Available from, as of March 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cbbb12f-760d-487d-b789-ae2d52a3e01f
Belviq is contraindicated during pregnancy, because weight loss offers no potential benefit to a pregnant woman and may result in fetal harm. Maternal exposure to lorcaserin in late pregnancy in rats resulted in lower body weight in offspring which persisted to adulthood. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard of maternal weight loss to the fetus.
US Natl Inst Health; DailyMed. Current Medication Information for BELVIQ (lorcaserin hydrochloride) tablets (August 2012). Available from, as of March 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cbbb12f-760d-487d-b789-ae2d52a3e01f
Patients who develop signs or symptoms of valvular heart disease, including dyspnea, dependent edema, congestive heart failure, or a new cardiac murmur while being treated with Belviq should be evaluated and discontinuation of Belviq should be considered.
US Natl Inst Health; DailyMed. Current Medication Information for BELVIQ (lorcaserin hydrochloride) tablets (August 2012). Available from, as of March 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cbbb12f-760d-487d-b789-ae2d52a3e01f
For more Drug Warnings (Complete) data for Lorcaserin (22 total), please visit the HSDB record page.
For the treatment of obesity, as an adjunct to a reduced-calorie diet and increased physical activity.
FDA Label
Treatment of obesity
Lorcaserin produced a dose-dependent weight loss over a 12-week period by promoting satiety and decreasing food consumption.
A - Alimentary tract and metabolism
A08 - Antiobesity preparations, excl. diet products
A08A - Antiobesity preparations, excl. diet products
A08AA - Centrally acting antiobesity products
A08AA11 - Lorcaserin
Absorption
Lorcaserin has a peak plasma concentration of about 1.5 - 2 hours, but the bioavailability was not determined.
Route of Elimination
Lorcaserin is eliminated by hepatic metabolism, and the metabolites are eliminated mostly in the urine (92.3%) and some through feces (2.2%).
Volume of Distribution
The volume of distribution was not determined, but lorcaserin distributes to the central nervous system and cerebrospinal fluid.
Clearance
The clearance value was not determined.
Lorcaserin distributes to the cerebrospinal fluid and central nervous system in humans. Lorcaserin hydrochloride is moderately bound (approximately 70%) to human plasma proteins.
US Natl Inst Health; DailyMed. Current Medication Information for BELVIQ (lorcaserin hydrochloride) tablets (August 2012). Available from, as of March 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cbbb12f-760d-487d-b789-ae2d52a3e01f
Lorcaserin is absorbed from the gastrointestinal tract with peak plasma concentration occurring 1.5 - 2 hours after oral dosing. The absolute bioavailability of lorcaserin has not been determined. Lorcaserin has a plasma half life of approximately 11 hours; steady state is reached within 3 days after twice daily dosing, and accumulation is estimated to be approximately 70%.
US Natl Inst Health; DailyMed. Current Medication Information for BELVIQ (lorcaserin hydrochloride) tablets (August 2012). Available from, as of March 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cbbb12f-760d-487d-b789-ae2d52a3e01f
Lorcaserin is extensively metabolized by the liver and the metabolites are excreted in the urine. In a human mass balance study in which healthy subjects ingested radiolabeled lorcaserin, 94.5% of radiolabeled material was recovered, with 92.3% and 2.2% recovered from urine and feces, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for BELVIQ (lorcaserin hydrochloride) tablets (August 2012). Available from, as of March 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cbbb12f-760d-487d-b789-ae2d52a3e01f
Lorcaserin has extensive hepatic metabolism producing inactive compounds. Lorcaserin sulfamate (M1) is the major metabolite circulating in the plasma, and N-carbamoyl glucuronide lorcaserin (M5) is the major metabolite in urine. Other minor metabolites that are both excreted in urine are glucuronide or sulfate conjugates.
Lorcaserin is extensively metabolized by the liver and the metabolites are excreted in the urine. In a human mass balance study in which healthy subjects ingested radiolabeled lorcaserin, 94.5% of radiolabeled material was recovered, with 92.3% and 2.2% recovered from urine and feces, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for BELVIQ (lorcaserin hydrochloride) tablets (August 2012). Available from, as of March 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cbbb12f-760d-487d-b789-ae2d52a3e01f
Lorcaserin is extensively metabolized in the liver by multiple enzymatic pathways. After oral administration of Belviq, the major circulating metabolite is lorcaserin sulfamate (M1), with a plasma Cmax that exceeds lorcaserin Cmax by 1- to 5-fold. N-carbamoyl glucuronide lorcaserin (M5) is the major metabolite in urine; M1 is a minor metabolite in urine, representing approximately 3% of dose. Other minor metabolites excreted in urine were identified as glucuronide or sulfate conjugates of oxidative metabolites. The principal metabolites exert no pharmacological activity at serotonin receptors.
US Natl Inst Health; DailyMed. Current Medication Information for BELVIQ (lorcaserin hydrochloride) tablets (August 2012). Available from, as of March 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cbbb12f-760d-487d-b789-ae2d52a3e01f
Lorcaserin, a selective serotonin 5-hydroxytryptamine 2C receptor agonist, is being developed for weight management. The oxidative metabolism of lorcaserin, mediated by recombinant human cytochrome P450 (P450) and flavin-containing monooxygenase (FMO) enzymes, was examined in vitro to identify the enzymes involved in the generation of its primary oxidative metabolites, N-hydroxylorcaserin, 7-hydroxylorcaserin, 5-hydroxylorcaserin, and 1-hydroxylorcaserin. Human CYP1A2, CYP2A6, CYP2B6, CYP2C19, CYP2D6, CYP3A4, and FMO1 are major enzymes involved in N-hydroxylorcaserin; CYP2D6 and CYP3A4 are enzymes involved in 7-hydroxylorcaserin; CYP1A1, CYP1A2, CYP2D6, and CYP3A4 are enzymes involved in 5-hydroxylorcaserin; and CYP3A4 is an enzyme involved in 1-hydroxylorcaserin formation. In 16 individual human liver microsomal preparations (HLM), formation of N-hydroxylorcaserin was correlated with CYP2B6, 7-hydroxylorcaserin was correlated with CYP2D6, 5-hydroxylorcaserin was correlated with CYP1A2 and CYP3A4, and 1-hydroxylorcaserin was correlated with CYP3A4 activity at 10.0 uM lorcaserin. No correlation was observed for N-hydroxylorcaserin with any P450 marker substrate activity at 1.0 uM lorcaserin. N-Hydroxylorcaserin formation was not inhibited by CYP1A2, CYP2A6, CYP2B6, CYP2C19, CYP2D6, and CYP3A4 inhibitors at the highest concentration tested. Furafylline, quinidine, and ketoconazole, selective inhibitors of CYP1A2, CYP2D6, and CYP3A4, respectively, inhibited 5-hydroxylorcaserin (IC(50) = 1.914 uM), 7-hydroxylorcaserin (IC(50) = 0.213 uM), and 1-hydroxylorcaserin formation (IC(50) = 0.281 uM), respectively. N-Hydroxylorcaserin showed low and high K(m) components in HLM and 7-hydroxylorcaserin showed lower K(m) than 5-hydroxylorcaserin and 1-hydroxylorcaserin in HLM. The highest intrinsic clearance was observed for N-hydroxylorcaserin, followed by 7-hydroxylorcaserin, 5-hydroxylorcaserin, and 1-hydroxylorcaserin in HLM. Multiple human P450 and FMO enzymes catalyze the formation of four primary oxidative metabolites of lorcaserin, suggesting that lorcaserin has a low probability of drug-drug interactions by concomitant medications.
PMID:22266842 Usmani KA et al; Drug Metab Dispos 40 (4): 761-71 (2012)
Lorcaserin, a selective serotonin 5-HT(2C) receptor agonist, is a weight management agent in clinical development. Lorcaserin N-carbamoyl glucuronidation governs the predominant excretory pathway of lorcaserin in humans. Human UDP-glucuronosyltransferases (UGTs) responsible for lorcaserin N-carbamoyl glucuronidation are identified herein. Lorcaserin N-carbamoyl glucuronide formation was characterized by the following approaches: metabolic screening using human tissues (liver, kidney, intestine, and lung) and recombinant enzymes, kinetic analyses, and inhibition studies. Whereas microsomes from all human tissues studied herein were found to be catalytically active for lorcaserin N-carbamoyl glucuronidation, liver microsomes were the most efficient. With recombinant UGT enzymes, lorcaserin N-carbamoyl glucuronidation was predominantly catalyzed by three UGT2Bs (UGT2B7, UGT2B15, and UGT2B17), whereas two UGT1As (UGT1A6 and UGT1A9) played a minor role. UGT2B15 was most efficient, with an apparent K(m) value of 51.6 + or - 1.9 uM and V(max) value of 237.4 + or - 2.8 pmol/mg protein/min. The rank order of catalytic efficiency of human UGT enzymes for lorcaserin N-carbamoyl glucuronidation was UGT2B15 > UGT2B7 > UGT2B17 > UGT1A9 > UGT1A6. Inhibition of lorcaserin N-carbamoyl glucuronidation activities of UGT2B7, UGT2B15, and UGT2B17 in human liver microsomes by mefenamic acid, bisphenol A, and eugenol further substantiated the involvement of these UGT2B isoforms. In conclusion, multiple human UGT enzymes catalyze N-carbamoyl glucuronidation of lorcaserin; therefore, it is unlikely that inhibition of any one of these UGT activities will lead to significant inhibition of the lorcaserin N-carbamoyl glucuronidation pathway. Thus, the potential for drug-drug interaction by concomitant administration of a drug(s) that is metabolized by any of these UGTs is remote.
PMID:22259019 Sadeque AJ et al; Drug Metab Dispos 40 (4): 772-8 (2012)
The plasma half life is approximately 11 hours.
Although the exact mechanism is unknown, it is believed to involve the selective activation of 5-HT2C receptors in the anorexigenic pro-opiomelanocortin neurons in the arcuate nucleus of the hypothalamus. This results in decreased food intake and satiety by promoting the release of alpha-melanocortin stimulating hormone, which acts on melanocortin-4 receptors.
Lorcaserin is believed to decrease food consumption and promote satiety by selectively activating 5-HT2C receptors on anorexigenic pro-opiomelanocortin neurons located in the hypothalamus. The exact mechanism of action is not known.
US Natl Inst Health; DailyMed. Current Medication Information for BELVIQ (lorcaserin hydrochloride) tablets (August 2012). Available from, as of March 13, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cbbb12f-760d-487d-b789-ae2d52a3e01f
In this study, /the researchers/ describe the in vitro and in vivo characteristics of lorcaserin ((1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3 benzazepine), a selective, high affinity 5-HT(2C) full agonist. Lorcaserin bound to human and rat 5-HT(2C) receptors with high affinity (K(i) = 15 +/- 1 nM, 29 +/- 7 nM, respectively), and it was a full agonist for the human 5-HT(2C) receptor in a functional inositol phosphate accumulation assay, with 18- and 104-fold selectivity over 5-HT(2A) and 5-HT(2B) receptors, respectively. Lorcaserin was also highly selective for human 5-HT(2C) over other human 5-HT receptors (5-HT(1A), 5-HT(3), 5-HT(4C), 5-HT5(5A), 5-HT(6), and 5-HT(7)), in addition to a panel of 67 other G protein-coupled receptors and ion channels. Lorcaserin did not compete for binding of ligands to serotonin, dopamine, and norepinephrine transporters, and it did not alter their function in vitro. Behavioral observations indicated that unlike the 5-HT(2A) agonist (+/-)-1-(2,5-dimethoxy-4-phenyl)-2-aminopropane, lorcaserin did not induce behavioral changes indicative of functional 5-HT(2A) agonist activity. Acutely, lorcaserin reduced food intake in rats, an effect that was reversed by pretreatment with the 5-HT(2C)-selective antagonist 6-chloro-5-methyl-1-[6-(2-methylpyridin-3-yloxy)pyridin-3-yl-carbamoyl]indoline (SB242,084) but not the 5-HT(2A) antagonist (R)-(+)-alpha-(2,3-dimethoxyphenyl)-1-(2-(4-fluorophenylethyl))-4-piperidine-methanol (MDL 100,907), demonstrating mediation by the 5-HT(2C) receptor. Chronic daily treatment with lorcaserin to rats maintained on a high fat diet produced dose-dependent reductions in food intake and body weight gain that were maintained during the 4-week study. Upon discontinuation, body weight returned to control levels. These data demonstrate lorcaserin to be a potent, selective, and efficacious agonist of the 5-HT(2C) receptor, with potential for the treatment of obesity.
PMID:18252809 Thomsen WJ et al; J Pharmacol Exp Ther. 2008 May;325(2):577-87 (2008)


