



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada
0

Australia

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
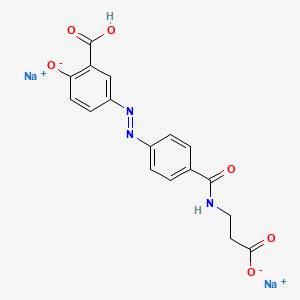

1. 5-(carboxyethylcarbamoyl-4-phenylazo)salicylic Acid
2. Balsalazide
3. Balsalazine
4. Bx 661a
5. Bx661a
6. Colazal
7. Colazide
1. Colazal
2. 213594-60-6
3. 82101-18-6
4. Balsalazide Sodium
5. Colazide
6. Bx-661a
7. Balsalazidedisodium
8. Balsalazide Disodium Anhydrous
9. 15asw03c9s
10. Balsalazide Disodium Salt Hydrate
11. Giazo
12. Disodium;3-[[4-[(3-carboxy-4-oxidophenyl)diazenyl]benzoyl]amino]propanoate
13. Balsalazine
14. Balsalazide Disodium [usan]
15. Bx 661a
16. Nsc-760046
17. Balzide
18. Balsalazide Disodium Salt
19. Unii-15asw03c9s
20. Schembl30360
21. Schembl3666408
22. Chembl1200760
23. Hms2097a22
24. Hms3264m06
25. Hms3714a22
26. Balsalazide Sodium [who-dd]
27. Akos015896484
28. Akos025294907
29. Ccg-213078
30. Benzoic Acid, 5-((4-(((2-carboxyethyl)amino)carbonyl)phenyl)azo)-2-hydroxy-, Disodium Salt
31. Disodium 5-((4-(((2-carboxyethyl)amino)carbonyl)phenyl)azo)-2-hydroxybenzoate
32. Disodium 3-[[4-[(2-carboxylatoethylamino)-oxomethyl]phenyl]hydrazinylidene]-6-oxo-1-cyclohexa-1,4-dienecarboxylate
33. Ft-0622551
34. Ft-0658348
35. Balsalazide Disodium Salt Anhydrous [mi]
36. A815288
37. J-014008
38. Q27251713
39. 5-[4-(2-carboxyethylcarbamoyl)phenylazo]salicylic Acid Disodium Salt
40. Sodium (e)-5-((4-((2-carboxylatoethyl)carbamoyl)phenyl)diazenyl)-2-hydroxybenzoate
41. Sodium(e)-5-((4-((2-carboxylatoethyl)carbamoyl)phenyl)diazenyl)-2-hydroxybenzoate
42. 5-[(1e)-[4-[[(2-carboxyethyl)amino]carbonyl]phenyl]azo]-2-hydroxybenzoic Acid Disodium Salt
43. Benzoic Acid, 5-((1e)-(4-(((2-carboxyethyl)amino)carbonyl)phenyl)azo)-2-hydroxy-, Disodium Salt
44. Benzoic Acid, 5-((1e)-2-(4-(((2-carboxyethyl)amino)carbonyl)phenyl)diazenyl)-2-hydroxy-, Sodium Salt (1:2)
45. Disodium 3-[[4-[(3-oxidanidyl-3-oxidanylidene-propyl)carbamoyl]phenyl]hydrazinylidene]-6-oxidanylidene-cyclohexa-1,4-diene-1-carboxylate
46. Disodium,(3z)-3-[[4-(2-carboxylatoethylcarbamoyl)phenyl]hydrazinylidene]-6-oxocyclohexa-1,4-diene-1-carboxylate
| Molecular Weight | 401.28 g/mol |
|---|---|
| Molecular Formula | C17H13N3Na2O6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 6 |
| Exact Mass | 401.05997371 g/mol |
| Monoisotopic Mass | 401.05997371 g/mol |
| Topological Polar Surface Area | 154 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 539 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Gastrointestinal Agents
Drugs used for their effects on the gastrointestinal system, as to control gastric acidity, regulate gastrointestinal motility and water flow, and improve digestion. (See all compounds classified as Gastrointestinal Agents.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)








