



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
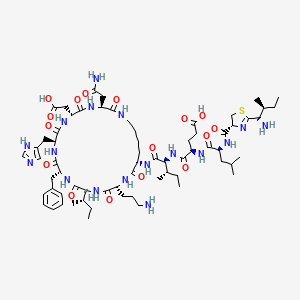

1. Bacitracin A2a
2. Baciguent
3. Fortracin
4. Bacitracinum
5. Parentracin
6. Penitracin
7. Topitracin
8. Zutracin
9. Baciim
10. Baci-rx
11. Solu-tracin 50
12. 22601-59-8
13. Chebi:35862
14. Dda3rrx0p7
15. Altracin
16. Bacitracin A1
17. E700
18. Bacitracin F, 1-(n-((2-(1-amino-2-methylbutyl)-4,5-dihydro-4-thiazolyl)carbonyl)-l-leucine)-
19. Ayfivin
20. Unii-dda3rrx0p7
21. Baciliquin
22. Bacilliquin
23. Bacitracina
24. Bacitracine
25. Mycitracin
26. Topitrasin
27. Tropitracin
28. Septa
29. Spectrocin Plus
30. Bacitek Ointment
31. Nsc-45737
32. Bacitracin Powder
33. Ak-tracin
34. Baci-jel
35. Bacitracin Complex
36. Nsc-755905
37. Baciferm 50
38. Bacitracin, Sterile
39. Einecs 245-115-9
40. Nsc 45737
41. Bacitracine [french]
42. Bacitracinum [latin]
43. Albac 50
44. Bacitracina [spanish]
45. Bactine Triple Antibiotic
46. Bacitracin(non-injectable)
47. Usaf Cb-7
48. Unii-58h6rwo52i
49. Mycitracin Plus Pain Reliever
50. 58h6rwo52i
51. Chembl1200558
52. Schembl20385900
53. Hsdb 6418
54. Bacitracin [usp:inn:ban:jan]
55. Einecs 215-786-2
56. Bdbm50458054
57. Bacitracin-neomycin-polymyxin Ointment
58. Db00626
59. Nsc 755905
60. Ai3-50147-x
61. Campho-phenique Triple Plus Pain Reliever
62. Q424319
63. Mycitracin Triple Antibiotic First Aid Ointment Maximum Strength
64. 85800-09-5
65. L-asparagine, N-(((4r)-2-((1s,2s)-1-amino-2-methylbutyl)-4,5-dihydro-4-thiazolyl)carbonyl)-l-leucyl-d-.alpha.-glutamyl-l-isoleucyl-l-lysyl-d-ornithyl-l-isoleucyl-d-phenylalanyl-l-histidyl-d-.alpha.-aspartyl-, (10->4)-lactam
66. N-({(4r)-2-[(1s,2s)-1-amino-2-methylbutyl]-4,5-dihydro-1,3-thiazol-4-yl}carbonyl)-l-leucyl-d-alpha-glutamyl-n-[(3s,6r,9s,12r,15s,18r,21s)-3-(2-amino-2-oxoethyl)-18-(3-aminopropyl)-12-benzyl-15-[(2s)-butan-2-yl]-6-(carboxymethyl)-9-(1h-imidazol-4-ylmethyl)-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptaazacyclopentacosan-21-yl]-l-isoleucinamide
| Molecular Weight | 1422.7 g/mol |
|---|---|
| Molecular Formula | C66H103N17O16S |
| XLogP3 | -4.1 |
| Hydrogen Bond Donor Count | 17 |
| Hydrogen Bond Acceptor Count | 21 |
| Rotatable Bond Count | 31 |
| Exact Mass | 1421.74894144 g/mol |
| Monoisotopic Mass | 1421.74894144 g/mol |
| Topological Polar Surface Area | 556 Ų |
| Heavy Atom Count | 100 |
| Formal Charge | 0 |
| Complexity | 2850 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 15 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Baciim |
| Drug Label | Bacitracin for Injection, USP is a sterile antibiotic for intramuscular administration. Bacitracin is derived from cultures of Bacillus subtilis (Tracey). It is a white to pale buff, hygroscopic powder, odorless or having a slight odor. It is freely... |
| Active Ingredient | Bacitracin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 50,000 units/vial |
| Market Status | Prescription |
| Company | X Gen Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Baciim |
| Drug Label | Bacitracin for Injection, USP is a sterile antibiotic for intramuscular administration. Bacitracin is derived from cultures of Bacillus subtilis (Tracey). It is a white to pale buff, hygroscopic powder, odorless or having a slight odor. It is freely... |
| Active Ingredient | Bacitracin |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 50,000 units/vial |
| Market Status | Prescription |
| Company | X Gen Pharms |
Bacitracin is indicated in topical formulations for acute and chronic localized skin infections. Occasionally, it is also used intramuscularly for infantile streptococcal pneumonia and empyema. Bacitracin is also formulated as an ointment with neomycin and polymyxin B for over the counter use. A bacitracin ointment formulated with neomycin and polymyxin B along with hydrocortisone is indicated for the treatment of corticosteroid responsive dermatoses with secondary infection.
Bacitracin is a mixture of polypeptides that prevent the formation of the bacterial cell wall and oxidatively cleave DNA. It has a short duration of action as it must be given every 3 to 4 hours topically. Bacitracin is nephrotoxic when given intramuscularly and may lead to renal failure.
Absorption
Topical, ophthalmic, and oral formulations of bacitracin are poorly absorbed systemically. Intramuscular bacitracin is readily and completely absorbed.
Route of Elimination
Bacitracin is mainly excreted renally with 87% of and intramuscular dose being recovered in the urine after 6 hours.
Volume of Distribution
Data regarding the volume of distribution of bacitracin in humans is not readily available.
Clearance
Data regarding the clearance of bacitracin in humans has not been well studied. A study of 9 subjects in 1947 shows a renal clearance of 105-283mL/min with an average renal clearance of 159mL/min.
Data regarding the metabolism of bacitracin in humans is not readily available. Because bacitracin is a protein it is expected to be metabolized into smaller polypeptides and amino acids. However, the structure of bacitracin may afford it some protection from the action of proteases.
Data regarding the half life of bacitracin in humans is not readily available.
Bacitracin binds to a divalent metal ion such as Mn(II), Co(II), Ni(II), Cu(II), or Zn(II). These complexes bind C55-isoprenyl pyrophosphate, preventing the hydrolysis of a lipid dolichol pyrophosphate, which finally inhibits cell wall synthesis. Bacitracin metal complexes also bind and oxidatively cleave DNA.






