



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada
0

Australia

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
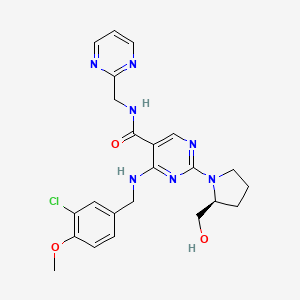

1. 4-((3-chloro-4-methoxybenzyl)amino)-2-(2-(hydroxymethyl)-1-pyrrolidinyl)-n-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide
2. Stendra
1. 330784-47-9
2. Stendra
3. Spedra
4. Ta 1790
5. Ta-1790
6. (s)-4-((3-chloro-4-methoxybenzyl)amino)-2-(2-(hydroxymethyl)pyrrolidin-1-yl)-n-(pyrimidin-2-ylmethyl)pyrimidine-5-carboxamide
7. Chebi:66876
8. Dr5s136ivo
9. (s)-2-(2-hydroxymethyl-1-pyrrolidinyl)-4-(3-chloro-4-methoxybenzylamino)-5-[(2-pyrimidinylmethyl)carbamoyl]pyrimidine
10. (s)-4-(3-chloro-4-methoxybenzylamino)-2-(2-hydroxymethylpyrrolidin-1-yl)-n-pyrimidin-2-ylmethyl-5-pyrimidinecarboxamide
11. (s)-4-[(3-chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]-n-(2pyrimidinylmethyl)-5-pyrimidinecarboxamide
12. 4-[(3-chloro-4-methoxybenzyl)amino]-2-[(2s)-2-(hydroxymethyl)pyrrolidin-1-yl]-n-(pyrimidin-2-ylmethyl)pyrimidine-5-carboxamide
13. 4-[(3-chloro-4-methoxyphenyl)methylamino]-2-[(2s)-2-(hydroxymethyl)pyrrolidin-1-yl]-n-(pyrimidin-2-ylmethyl)pyrimidine-5-carboxamide
14. 4-((3-chloro-4-methoxybenzyl)amino)-2-((2s)-2-(hydroxymethyl)pyrrolidin-1-yl)-n-(pyrimidin-2-ylmethyl)pyrimidine-5-carboxamide
15. 5-pyrimidinecarboxamide, 4-(((3-chloro-4-methoxyphenyl)methyl)amino)-2-((2s)-2-(hydroxymethyl)-1-pyrrolidinyl)-n-(2-pyrimidinylmethyl)-
16. Stendra (tn)
17. Avanafil [usan:inn]
18. Unii-dr5s136ivo
19. Zepeed
20. Ine-5-carboxamide
21. Spedra (tn)
22. Avanafil [usan]
23. Avanafil (usan/inn)
24. Avanafil [inn]
25. Avanafil [mi]
26. Avanafil [vandf]
27. Avanafil [mart.]
28. Avanafil [who-dd]
29. Avanafil 100 Microg/ml In Acetonitrile:dimethylsulfoxide
30. Schembl118799
31. Avanafil [orange Book]
32. Gtpl7448
33. Chembl1963681
34. Amy1794
35. Dtxsid50186727
36. 4-((3-chloro-4-methoxybenzyl)amino)-2-(2-(hydroxymethyl)-1-pyrrolidinyl)-n-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide
37. Bdbm50036629
38. Cs1566
39. Mfcd11977961
40. S4019
41. Ta1790
42. Zinc11677857
43. Akos024462448
44. Ccg-229896
45. Db06237
46. Vi-0162
47. Ncgc00386241-01
48. As-20106
49. Hy-18252
50. Sw219217-1
51. D03217
52. Ab01565827_02
53. J-019006
54. Q2873270
55. Brd-k65781196-001-01-4
56. (s)-2-(2-hydroxymethyl-1-pyrrolidinyl)-4-(3-chloro-4-methoxybenzylamino)-5-[n-(2-pyrimidylmethyl)carbamoyl]-pyrimidine
57. (s)-4-[(3-chlor-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1- Pyrrolidinyl]-n-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamid
58. 4-[(3-chloranyl-4-methoxy-phenyl)methylamino]-2-[(2s)-2-(hydroxymethyl)pyrrolidin-1-yl]-n-(pyrimidin-2-ylmethyl)pyrimid
59. 4-[(3-chloranyl-4-methoxy-phenyl)methylamino]-2-[(2s)-2-(hydroxymethyl)pyrrolidin-1-yl]-n-(pyrimidin-2-ylmethyl)pyrimid Ine-5-carboxamide
60. Avanafil; (s)-4-chloro-6-((3-chloro-4-methoxybenzyl)amino)-2-(2-(hydroxymethyl)pyrrolidin-1-yl)-n-(pyrimidin-2-ylmethyl)pyrimidine-5-carboxamide
61. E6l
| Molecular Weight | 483.9 g/mol |
|---|---|
| Molecular Formula | C23H26ClN7O3 |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 9 |
| Exact Mass | 483.1785654 g/mol |
| Monoisotopic Mass | 483.1785654 g/mol |
| Topological Polar Surface Area | 125 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 642 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Stendra |
| PubMed Health | Avanafil (By mouth) |
| Drug Classes | Erectile Dysfunction Agent |
| Drug Label | STENDRA (avanafil) is a selective inhibitor of cGMP-specific PDE5.Avanafil is designated chemically as (S)-4-[(3-Chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide and has the following... |
| Active Ingredient | Avanafil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Vivus |
| 2 of 2 | |
|---|---|
| Drug Name | Stendra |
| PubMed Health | Avanafil (By mouth) |
| Drug Classes | Erectile Dysfunction Agent |
| Drug Label | STENDRA (avanafil) is a selective inhibitor of cGMP-specific PDE5.Avanafil is designated chemically as (S)-4-[(3-Chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide and has the following... |
| Active Ingredient | Avanafil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 100mg; 50mg |
| Market Status | Prescription |
| Company | Vivus |
Avanafil is indicated for the treatment of erectile dysfunction.
FDA Label
Treatment of erectile dysfunction in adult men.
In order for Spedra to be effective, sexual stimulation is required.
Avanafil is a strong competitive inhibitor of phosphodiesterase 5 (PDE5) with a demonstrated _in vitro_ IC50 of 5.2 nM. Its inhibitory effects on PDE5 are 100-fold more potent than on PDE6 and >1000-fold more potent than on other PDE enzymes, meaning it is less likely to cause visual disturbances and cardiovascular adverse effects when compared with less selective PDE5 inhibitors such as [sildenafil] and [vardenafil]. It has a relatively quick onset of action allowing for administration as early as 15 minutes prior to sexual activity. PDE5 inhibitors like avanafil can cause significant drug interactions when administered alongside certain antihypertensive agents (e.g. alpha blockers, substantial amounts of alcohol). PDE5 inhibitors have also been associated with the development of non-arteritic anterior ischemic optic neuropathy (NAION), a rare condition that typically presents as sudden loss of vision in one or both eyes and appears to be more common in patients with a "crowded" optic disc. Patients presenting with any degree of vision loss should immediately discontinue use of all PDE5 inhibitors and seek medical attention. In some jurisdictions, a history of NAION or other degenerative retinal disorders is considered a contraindication to avanafil therapy.
G04BE10
G04BE10
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G04 - Urologicals
G04B - Urologicals
G04BE - Drugs used in erectile dysfunction
G04BE10 - Avanafil
Absorption
Avanafil is rapidly absorbed following oral administration (Tmax of 30-45 minutes) and appears to have low to moderate oral bioavailability, though formal studies have not been conducted. Administration with a meal results in a mean delay in Tmax of 1.12 to 1.25 hours, a 39% mean reduction in Cmax, and a negligible effect on AUC.
Route of Elimination
Following oral administration, avanafil is extensively metabolized. Approximately 62% of a given dose is excreted as metabolites in the feces and approximately 21% as metabolites in the urine.
Volume of Distribution
The apparent volume of distribution of avanafil is 47 to 83 L.
Avanafil is extensively metabolized, primarily by CYP3A4 and to a lesser extent by CYP2C9. There are two major metabolites formed, M4 and M16, which exist in the plasma at concentrations 23% and 29% that of the parent compound, respectively. The M16 metabolite lacks pharmacologic effect, but the M4 metabolite has an inhibitory potency for PDE5 18% that of avanafil and accounts for approximately 4% of the observed pharmacologic activity of avanafil.
Studies have demonstrated variability in the terminal elimination half-life of avanafil, with estimates ranging between 5 - 17 hours.
Avanafil inhibits the cGMP-specific phosphodiesterase type 5 (PDE5) which is responsible for the degradation of cGMP in the corpus cavernosum located around the penis. Sexual arousal results in the local release of nitric oxide, which in turn stimulates the enzyme guanylate cyclase to produce cGMP. Elevated levels of cGMP result in local smooth muscle relaxation and increased blood flow to the penis (i.e. an erection). As PDE5 inhibitors like avanafil require the endogenous release of nitric oxide in order to exert their pharmacologic effect, they have no effect on the user in the absence of sexual stimulation/arousal.








