
Synopsis
Synopsis
0
NDC API
0
VMF
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
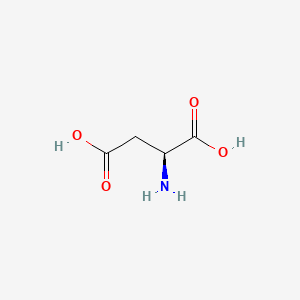

1. (+-)-aspartic Acid
2. (r,s)-aspartic Acid
3. Ammonium Aspartate
4. Aspartate
5. Aspartate Magnesium Hydrochloride
6. Aspartate, Ammonium
7. Aspartate, Calcium
8. Aspartate, Dipotassium
9. Aspartate, Disodium
10. Aspartate, Magnesium
11. Aspartate, Monopotassium
12. Aspartate, Monosodium
13. Aspartate, Potassium
14. Aspartate, Sodium
15. Aspartic Acid, Ammonium Salt
16. Aspartic Acid, Calcium Salt
17. Aspartic Acid, Dipotassium Salt
18. Aspartic Acid, Disodium Salt
19. Aspartic Acid, Hydrobromide
20. Aspartic Acid, Hydrochloride
21. Aspartic Acid, Magnesium (1:1) Salt, Hydrochloride, Trihydrate
22. Aspartic Acid, Magnesium (2:1) Salt
23. Aspartic Acid, Magnesium-potassium (2:1:2) Salt
24. Aspartic Acid, Monopotassium Salt
25. Aspartic Acid, Monosodium Salt
26. Aspartic Acid, Potassium Salt
27. Aspartic Acid, Sodium Salt
28. Calcium Aspartate
29. Dipotassium Aspartate
30. Disodium Aspartate
31. L Aspartate
32. L Aspartic Acid
33. L-aspartate
34. L-aspartic Acid
35. Magnesiocard
36. Magnesium Aspartate
37. Mg-5-longoral
38. Monopotassium Aspartate
39. Monosodium Aspartate
40. Potassium Aspartate
41. Sodium Aspartate
1. L-aspartic Acid
2. 56-84-8
3. H-asp-oh
4. Asparagic Acid
5. (2s)-2-aminobutanedioic Acid
6. L-aspartate
7. Aspartate
8. Aspatofort
9. (s)-2-aminosuccinic Acid
10. L-asparagic Acid
11. L-aminosuccinic Acid
12. Asparaginic Acid
13. L-asparaginic Acid
14. (s)-aspartic Acid
15. (2s)-aspartic Acid
16. (s)-aminobutanedioic Acid
17. Aspartic Acid, L-
18. L-aspartinsaeure
19. L-asparaginsaeure
20. L-asparaginsyra
21. Acidum Asparticum
22. L-(+)-aspartic Acid
23. L-asp
24. L-2-aminobutanedioic Acid
25. 25608-40-6
26. Aspartate, L-
27. Asparaginsaeure [german]
28. Aminosuccinic Acid
29. Butanedioic Acid, Amino-, (s)-
30. Aspartic Acid (van)
31. Asparagic Acid (van)
32. (s)-(+)-aminosuccinic Acid
33. Asparaginic Acid (van)
34. Acide Aspartique [inn-french]
35. Acido Aspartico [inn-spanish]
36. (l)-aspartic Acid
37. (+)-aspartic Acid
38. Fema No. 3565
39. Ccris 6181
40. Acidum Asparticum [inn-latin]
41. Hsdb 1430
42. L( )-aminobernsteinsaeure
43. Ai3-04461
44. Poly-l-aspartate
45. L-aspartic Acid Homopolymer
46. Nsc 3973
47. 6899-03-2
48. 2-aminosuccinic Acid
49. Deamidated Asparagine
50. Brn 1723530
51. Beta-l-aspartic Acid
52. Chebi:17053
53. Mfcd00002616
54. 30kyc7miai
55. Asp
56. (s)-(+)-aspartic Acid
57. (s)-2-aminobutanedioic Acid
58. Chembl274323
59. Fema No. 3656
60. Asparaginsaeure
61. (s)-aspartate
62. L-asparticacid
63. (+)-aspartate
64. Polyaspartic Acid
65. L-aspartic Acid-13c4
66. 1-amino-1,2-carboxyethane
67. (s)-aminobutanedioate
68. L(+)-aspartic Acid
69. 55443-54-4
70. Acido Aspartico
71. Acide Aspartique
72. Aspartic Acid [usan:inn]
73. (s)-aminosuccinic Acid
74. 4-04-00-02998 (beilstein Handbook Reference)
75. 39162-75-9
76. Einecs 200-291-6
77. Unii-30kyc7miai
78. L(+)-aminobernsteinsaeure
79. Aminosuccinate
80. Asparagate
81. Asparatate
82. L-aspartic Acid, Homopolymer
83. Nsc-3973
84. Aspartic Acid,l
85. L-aspartic-acid
86. L-asparagate
87. L-aminosuccinate
88. Aspartic Acid [usan:usp:inn]
89. (l)-aspartate
90. L- Aspartic Acid
91. Alpha-aminosuccinate
92. (2s)-aspartate
93. Poly-l-asparticacid
94. L-(+)-aspartate
95. [3h]-l-aspartate
96. L-[14c]aspartate
97. L-aspartic Acid, 2
98. [3h]l-aspartic Acid
99. (r)-2-aminosuccinate
100. (s)-2-aminosuccinate
101. Tocris-0214
102. [3h]-l-asp
103. (s)-amino-butanedioate
104. Alpha-aminosuccinic Acid
105. (s)-(+)-aspartate
106. [3h]-l-aspartic Acid
107. L-aspartic Acid (9ci)
108. 2-amino-3-methylsuccinate
109. Biomol-nt_000168
110. Bmse000031
111. Bmse000875
112. Aspartic Acid (usp/inn)
113. Ec 200-291-6
114. Aspartic Acid [ii]
115. Aspartic Acid [mi]
116. L-aspartic Acid (jp17)
117. Schembl3231
118. (s)-amino-butanedioic Acid
119. Aspartic Acid [inn]
120. L-aspartic Acid-[13c4]
121. L-aspartic Acid, >=98%
122. L-aspartic Acid, 99.0%
123. Lopac0_000133
124. Aspartic Acid [hsdb]
125. Aspartic Acid [inci]
126. Aspartic Acid [usan]
127. Aspartic Acid, L- (8ci)
128. Aspartic Acid [vandf]
129. Aspartic Acid [mart.]
130. L-aspartic Acid (h-asp-oh)
131. L-aspartic Acid [fcc]
132. L-aspartic Acid [jan]
133. (2s)-2-azanylbutanedioic Acid
134. Bpbio1_001128
135. Gtpl3309
136. Gtpl4534
137. Aspartic Acid [usp-rs]
138. Aspartic Acid [who-dd]
139. L-aspartic Acid [fhfi]
140. Dtxsid7022621
141. Aspartic Acid (l-aspartic Acid)
142. Bdbm18125
143. L-aspartic Acid, >=98%, Fg
144. Hms3260k08
145. Zinc895032
146. .alpha.-aminosuccinic Acid, (l)-
147. Aspartic Acid [ep Impurity]
148. Hy-n0666
149. Str04614
150. Tox21_500133
151. Aspartic Acid [ep Monograph]
152. Pdsp1_000819
153. Pdsp2_000806
154. Aspartic Acid [usp Monograph]
155. Akos006239578
156. Akos015853957
157. Am81585
158. Ccg-204228
159. Db00128
160. Lp00133
161. Sdccgsbi-0050121.p002
162. Alanine Impurity A [ep Impurity]
163. L-aspartic Acid (h-asp-oh) Usp Grade
164. Ncgc00024499-01
165. Ncgc00024499-02
166. Ncgc00024499-03
167. Ncgc00024499-04
168. Ncgc00024499-05
169. Ncgc00024499-06
170. Ncgc00024499-10
171. Ncgc00260818-01
172. Bp-13291
173. L-aspartic Acid, Vetec(tm) Reagent Grade
174. Db-029944
175. A0546
176. Cs-0009701
177. Eu-0100133
178. L-aspartic Acid, Bioxtra, >=99% (hplc)
179. S5632
180. L-aspartic Acid, Bioultra, >=99.5% (t)
181. A 9256
182. A-9220
183. C00049
184. D00013
185. D70832
186. Lysine Acetate Impurity A [ep Impurity]
187. M03000
188. L-aspartic Acid, Saj Special Grade, >=99.0%
189. A817928
190. A824434
191. L-aspartic Acid, Reagent Grade, >=98% (hplc)
192. Q178450
193. Sr-01000597734
194. Sr-01000597734-3
195. F8889-8684
196. Z1270403519
197. A4b5fb11-a4b6-4d75-9860-2acf670700b9
198. Aspartic Acid, European Pharmacopoeia (ep) Reference Standard
199. L-aspartic Acid, Certified Reference Material, Tracecert(r)
200. Aspartic Acid, United States Pharmacopeia (usp) Reference Standard
201. L-aspartic Acid, Bioreagent, Suitable For Cell Culture, Suitable For Insect Cell Culture
202. L-aspartic Acid, Pharmaceutical Secondary Standard; Certified Reference Material
203. L-aspartic Acid, From Non-animal Source, Meets Ep, Usp Testing Specifications, Suitable For Cell Culture, 98.5-101.0%
204. L-aspartic Acid, Pharmagrade, Ajinomoto, Ep, Jp, Usp, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production, Suitable For Cell Culture
| Molecular Weight | 133.10 g/mol |
|---|---|
| Molecular Formula | C4H7NO4 |
| XLogP3 | -2.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 133.03750770 g/mol |
| Monoisotopic Mass | 133.03750770 g/mol |
| Topological Polar Surface Area | 101 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 133 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
MEDICATION (VET): TO REDUCE AMMONIA BLOOD LEVELS & SAID TO BE OF VALUE IN OVERCOMING FATIGUE. ... DOSAGE: GIVEN ORALLY OR AS FEED ADDITIVE AGAINST STRESS INDUCED HIGH BLOOD AMMONIA LEVELS IN POULTRY & AGAINST AMMONIA INTOXICATED RATS. /ASPARTIC ACID/
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 28
Parenteral nutrition
Gerhartz, W. (exec ed.). Ullmann's Encyclopedia of Industrial Chemistry. 5th ed.Vol A1: Deerfield Beach, FL: VCH Publishers, 1985 to Present., p. VA2 84
/EXPL/: L-aspartate is a glycogenic amino acid, and it can also promote energy production via its metabolism in the Krebs cycle. These latter activities were the rationale for the claim that supplemental aspartate has an anti-fatigue effect on skeletal muscle, a claim that was never confirmed. /L-aspartate/
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ (2001) p.255
There are claims that L-aspartate is a special type of mineral transporter for cations, such as magnesium, into cells. Magnesium aspartate has not been found to be more biologically effective when compared with other magnesium salts. There are also claims that L-aspartate has ergogenic effects, that it enhances performance in both prolonged exercise and short intensive exercise. It is hypothesized that L-aspartate, especially the potassium magnesium aspartate salt, spares stores of muscle glycogen and/or promotes a faster rate of glycogen resynthesis during exercise. It has also been hypothesized that L-aspartate can enhance short intensive exercise by serving as a substrate for energy production in the Krebs cycle and for stimulating the purine nucleotide cycle. An animal study using injected aspartate failed to find any evidence of a glycogen-sparing effect or any ergogenic effects whatsoever. A more recent double-blind human study of male weight trainers similarly found aspartate supplementation to have no effect, and another study of the effect of aspartate on short intensive exercise again found no effect. /L-aspartate/
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ (2001) p.254
For more Therapeutic Uses (Complete) data for (L)-ASPARTIC ACID (6 total), please visit the HSDB record page.
Mild gastrointestinal side effects including diarrhea have been reported. /L-aspartate/
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ (2001) p.255
Because of lack of long-term safety studies, L-aspartate salts should be avoided by children, pregnant women and lactating women. /L-Aspartate/
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ (2001) p.255
The effects of oral administration of potassium and magnesium aspartate (K + Mg Asp) on physiologic responses to 90 min of treadmill walking at approximately 62% VO2 max were evaluated in seven healthy males (VO2 max = 59.5 ml X kg-1 X min-1). A total of 7.2 g of K + Mg Asp were administered to each subject during a 24 h period prior to work and compared to control and placebo trials. For control, placebo, and K + Mg Asp trials, no significant differences were observed in resting or exercise values for ventilation (VE), oxygen uptake (VO2), carbon dioxide production (VCO2), respiratory exchange ratio (RO), heart rate (HR), or blood pressure (BP). In addition, there were no differences between the three trials for exercise-induced decreases in body weight and increases in rectal temperature, or for pre- and post-exercise alterations in serum lactic acid, creatine kinase, lactic dehydrogenase, and percentage change in plasma volume. The findings from this study indicate that oral ingestion of K+ Mg Asp prior to exercise had no effect on cardiorespiratory, hematologic, and metabolic responses to 90 min of work conducted at approximately 62% VO2 max. /Potassium magnesium aspartate/
PMID:7138632 Hagan RD et al; Int J Sports Med 3 (3): 177-81 (1982)
This study examined the effects of aspartate supplementation (ASP) on plasma ammonia concentrations (NH4+) during and after a resistance training workout (RTW). Twelve male weight trainers were randomly administered ASP or vitamin C in a crossover, double blind protocol, each trial separated by 1 wk. ASP and vitamin C were given over a 2 hr period beginning 5 hr prior to the RTW. The RTW consisted of bench, incline, shoulder, and triceps presses, and biceps curls at 70% of one repetition maximum (1-RM). After the RTW a bench press test (BPT) to failure at 65% of 1-RM was used to assess performance. (NH4+) was determined preexercise, 20 and 40 min midworkout, immediately postexercise, and 15 min postexercise. Treatment-by-time ANOVAs, paired t tests, and contrast comparisons were used to identify mean differences. No significant differences were observed between treatments for (NH4+) or BPT. (NH4+) increased significantly from Pre to immediately postexercise for both the ASP and vitamin C trials. Acute ASP supplementation does not reduce (NH4+) during and after a high intensity RTW in weight trained subjects. /Potassium magnesium aspartate/
PMID:7670449 Tuttle JL et al; Int J Sport Nutr 5 (2): 102-9 (1995)
There is no support for the claim that aspartates are exercise performance enhancers, i.e. ergogenic aids.
L-aspartate is considered a non-essential amino acid, meaning that, under normal physiological conditions, sufficient amounts of the amino acid are synthesized in the body to meet the body's requirements. L-aspartate is formed by the transamination of the Krebs cycle intermediate oxaloacetate. The amino acid serves as a precursor for synthesis of proteins, oligopeptides, purines, pyrimidines, nucleic acids and L-arginine. L-aspartate is a glycogenic amino acid, and it can also promote energy production via its metabolism in the Krebs cycle. These latter activities were the rationale for the claim that supplemental aspartate has an anti-fatigue effect on skeletal muscle, a claim that was never confirmed.
Absorption
Absorbed from the small intestine by an active transport process
ASPARTIC ACID PLASMA CONCN WAS ELEVATED 30 MIN AFTER 1 G/KG L-ASPARTATE (ORAL OR IP) TO 15 DAY OLD & ADULT MICE. THEREAFTER, CONCN DECLINED EXPONENTIALLY WITH T/2 OF 0.2 HR IN BOTH. PLASMA CONCN NOT APPRECIABLY ALTERED BY 10 & 100 MG/KG L-ASPARTATE ORAL OR IP ADMIN.
OPPERMANN ET AL; J ENVIRON PATHOL TOXICOL 2(4) 987 (1979)
Following ingestion, L-aspartate is absorbed from the small intestine by an active transport process. Following absorption, L-aspartate enters the portal circulation and from there is transported to the liver, where much of it is metabolized to protein, purines, pyrimidines and L-arginine, and is catabolized as well. L-aspartate is not metabolized in the liver; it enters the systemic circulation, which distributes it to various tissues of the body. The cations associated with L-aspartate independently interact with various substances in the body and participate in various physiological processes. /L-aspartate/
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ (2001) p.254
... Contents of D- and L-aspartic acids in rats at different stages of growth (from 1 day before birth to 90 days after birth) were determined. D-Aspartic acid was detected in all the brain tissue samples tested, but at different levels. In the cerebrum of rats 1 day before birth, D-aspartic acid was found to be at the highest concentration of 81 nmol/g wet tissue. The level of D-aspartic acid in rat brain falls rapidly after birth, while the L-aspartic acid level increases with age.
PMID:11589464 Zhao S et al; J Chromatogr B Biomed Sci Appl 762 (1): 97-101 (2001)
Enzymatic synthesis of 11C-(4)-L-aspartic acid was undertaken using commercially available wheat germ phosphoenolpyruvate carboxylase. Whole-body distribution of the radioactive compound in rats showed higher accumulation in the salivary gland, glandular stomach and the pancreas, as well as in the lungs. Within 60 minutes after intravenous injection of 11C-(4)-L-aspartic acid, about 60% is removed as 11CO2 by expiration, indicating that the carbon atom at the fourth position of the radioactive compound is easily subjected to decarboxylation.
PMID:6494491 Nakamura T et al; Radioisotopes 33 (6): 363-9 (1984)
The brain efflux index method has been used to clarify the mechanism of efflux transport of acidic amino acids such as L-aspartic acid (L-Asp), L-glutamic acid (L-Glu), and D-aspartic acid (D-Asp) across the blood-brain barrier (BBB). About 85% of L-[3H]Asp and 40% of L-(3H)Glu was eliminated from the ipsilateral cerebrum within, respectively, 10 and 20 min of microinjection into the brain. The efflux rate constant of L-(3H)Asp and L-(3H)Glu was 0.207 and 0.0346 min(-1), respectively. However, D-(3H)Asp was not eliminated from brain over a 20-min period. The efflux of L-(3H)Asp and L-(3H)Glu was inhibited in the presence of excess unlabeled L-Asp and L-Glu, whereas D-Asp did not inhibit either form of efflux transport. Aspartic acid efflux across the BBB appears to be stereospecific. Using a combination of TLC and the bioimaging analysis, attempts were made to detect the metabolites of L-(3H)Asp and L-(3H)Glu in the ipsilateral cerebrum and jugular vein plasma following a microinjection into parietal cortex, area 2. Significant amounts of intact L-(3H)Asp and L-(3H)Glu were found in all samples examined, including jugular vein plasma, providing direct evidence that at least a part of the L-Asp and L-Glu in the brain interstitial fluid is transported across the BBB in the intact form. To compare the transport of acidic amino acids using brain parenchymal cells, brain slice uptake studies were performed. Although the slice-to-medium ratio of D-(3H)Asp was the highest, followed by L-[3H]Glu and L-[3H]Asp, the initial uptake rate did not differ for both L-(3H)Asp and D-(3H)Asp, suggesting that the uptake of aspartic acid in brain parenchymal cells is not stereospecific. These results provide evidence that the BBB may act as an efflux pump for L-Asp and L-Glu to reduce the brain interstitial fluid concentration and act as a static wall for D-Asp.
PMID:10461913 Hosoya K et al; J Neurochem 73 (3): 1206-11 (1999)
FOR L-ASPARTIC ACID, OXALOACETIC ACID IS PRODUCT OF OXIDATIVE DEAMINATION OR TRANSAMINATION; ALPHA-ALANINE IS PRODUCT OF DECARBOXYLATION. /FROM TABLE/
Fenaroli's Handbook of Flavor Ingredients. Volume 2. Edited, translated, and revised by T.E. Furia and N. Bellanca. 2nd ed. Cleveland: The Chemical Rubber Co., 1975., p. 829
METABOLIC PATHWAYS & PRODUCTS /IN ANIMAL BODY/: ASPARTIC ACID + CARBAMYLPHOSPHATE /PRODUCE/ PHOSPHORUS + CARBAMYLASPARTIC ACID GIVE PYRIMIDINES; ASPARTIC ACID /PRODUCES/ FUMARIC ACID + NH3; ASPARTIC ACID /PRODUCES/ ASPARTIC SEMIALDEHYDE /PRODUCES/ HOMOSERINE /PRODUCES/ (I) THREONINE, (II) METHIONINE, OR (III) LYSINE... /FROM TABLE/
Fenaroli's Handbook of Flavor Ingredients. Volume 2. Edited, translated, and revised by T.E. Furia and N. Bellanca. 2nd ed. Cleveland: The Chemical Rubber Co., 1975., p. 829
METABOLIC PATHWAYS & PRODUCTS /IN ANIMAL BODY/: ASPARTIC ACID GIVES NITROGEN OF PURINE RING...ASPARTIC ACID + IMP GIVE ADENYLOSUCCINATE GIVES AMP + FUMARATE... /FROM TABLE/
Fenaroli's Handbook of Flavor Ingredients. Volume 2. Edited, translated, and revised by T.E. Furia and N. Bellanca. 2nd ed. Cleveland: The Chemical Rubber Co., 1975., p. 829
Following ingestion, L-aspartate is absorbed from the small intestine by an active transport process. Following absorption, L-aspartate enters the portal circulation and from there is transported to the liver, where much of it is metabolized to protein, purines, pyrimidines and L-arginine, and is catabolized as well. D-aspartate is not metabolized in the liver; it enters the systemic circulation, which distributes it to various tissues of the body. The cations associated with L-aspartate independently interact with various substances in the body and participate in various physiological processes. /L-aspartate; D-aspartate/
Physicians Desk Reference (PDR) for Nutritional Supplements 1st ed, Medical Economics, Thomson Healthcare; Montvale, NJ (2001) p.254
There are also claims that L-aspartate has ergogenic effects, that it enhances performance in both prolonged exercise and short intensive exercise. It is hypothesized that L-aspartate, especially the potassium magnesium aspartate salt, spares stores of muscle glycogen and/or promotes a faster rate of glycogen resynthesis during exercise. It has also been hypothesized that L-aspartate can enhance short intensive exercise by serving as a substrate for energy production in the Krebs cycle and for stimulating the purine nucleotide cycle.
Global Sales Information

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
47
PharmaCompass offers a list of Aspartic Acid API API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Aspartic Acid API manufacturer or Aspartic Acid API supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Aspartic Acid API manufacturer or Aspartic Acid API supplier.
PharmaCompass also assists you with knowing the Aspartic Acid API API Price utilized in the formulation of products. Aspartic Acid API API Price is not always fixed or binding as the Aspartic Acid API Price is obtained through a variety of data sources. The Aspartic Acid API Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Aspartic Acid API manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Aspartic Acid API, including repackagers and relabelers. The FDA regulates Aspartic Acid API manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Aspartic Acid API API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Aspartic Acid API manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Aspartic Acid API supplier is an individual or a company that provides Aspartic Acid API active pharmaceutical ingredient (API) or Aspartic Acid API finished formulations upon request. The Aspartic Acid API suppliers may include Aspartic Acid API API manufacturers, exporters, distributors and traders.
click here to find a list of Aspartic Acid API suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Aspartic Acid API DMF (Drug Master File) is a document detailing the whole manufacturing process of Aspartic Acid API active pharmaceutical ingredient (API) in detail. Different forms of Aspartic Acid API DMFs exist exist since differing nations have different regulations, such as Aspartic Acid API USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Aspartic Acid API DMF submitted to regulatory agencies in the US is known as a USDMF. Aspartic Acid API USDMF includes data on Aspartic Acid API's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Aspartic Acid API USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Aspartic Acid API suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Aspartic Acid API Drug Master File in Japan (Aspartic Acid API JDMF) empowers Aspartic Acid API API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Aspartic Acid API JDMF during the approval evaluation for pharmaceutical products. At the time of Aspartic Acid API JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Aspartic Acid API suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Aspartic Acid API Drug Master File in Korea (Aspartic Acid API KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Aspartic Acid API. The MFDS reviews the Aspartic Acid API KDMF as part of the drug registration process and uses the information provided in the Aspartic Acid API KDMF to evaluate the safety and efficacy of the drug.
After submitting a Aspartic Acid API KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Aspartic Acid API API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Aspartic Acid API suppliers with KDMF on PharmaCompass.
A Aspartic Acid API CEP of the European Pharmacopoeia monograph is often referred to as a Aspartic Acid API Certificate of Suitability (COS). The purpose of a Aspartic Acid API CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Aspartic Acid API EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Aspartic Acid API to their clients by showing that a Aspartic Acid API CEP has been issued for it. The manufacturer submits a Aspartic Acid API CEP (COS) as part of the market authorization procedure, and it takes on the role of a Aspartic Acid API CEP holder for the record. Additionally, the data presented in the Aspartic Acid API CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Aspartic Acid API DMF.
A Aspartic Acid API CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Aspartic Acid API CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Aspartic Acid API suppliers with CEP (COS) on PharmaCompass.
A Aspartic Acid API written confirmation (Aspartic Acid API WC) is an official document issued by a regulatory agency to a Aspartic Acid API manufacturer, verifying that the manufacturing facility of a Aspartic Acid API active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Aspartic Acid API APIs or Aspartic Acid API finished pharmaceutical products to another nation, regulatory agencies frequently require a Aspartic Acid API WC (written confirmation) as part of the regulatory process.
click here to find a list of Aspartic Acid API suppliers with Written Confirmation (WC) on PharmaCompass.
Aspartic Acid API Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Aspartic Acid API GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Aspartic Acid API GMP manufacturer or Aspartic Acid API GMP API supplier for your needs.
A Aspartic Acid API CoA (Certificate of Analysis) is a formal document that attests to Aspartic Acid API's compliance with Aspartic Acid API specifications and serves as a tool for batch-level quality control.
Aspartic Acid API CoA mostly includes findings from lab analyses of a specific batch. For each Aspartic Acid API CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Aspartic Acid API may be tested according to a variety of international standards, such as European Pharmacopoeia (Aspartic Acid API EP), Aspartic Acid API JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Aspartic Acid API USP).

