



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
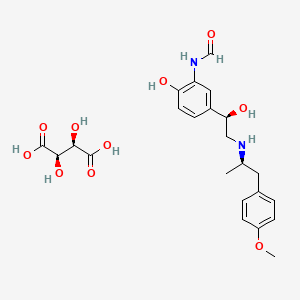

1. 200815-49-2
2. Brovana
3. (r,r)-arformoterol Tartrate
4. Arformoterol Tartrate [usan]
5. 5p8vj2i235
6. Arformoterol Tartrate (usan)
7. Formamide, N-[2-hydroxy-5-[(1r)-1-hydroxy-2-[[(1r)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]-, (2r,3r)-2,3-dihydroxybutanedioate (1:1)
8. (r,r)-formoterol Tartrate
9. (-)-n-(2-hydroxy-5-((1r)-1-hydroxy-2-(((1r)-2-(4-methoxyphenyl)-1-methylethyl)amino)ethyl)phenyl)formamide Hydrogen (2r,3r)-2,3-dihydroxybutanedioate (salt)
10. Formamide, N-(2-hydroxy-5-((1r)-1-hydroxy-2-(((1r)-2-(4-methoxyphenyl)-1-methylethyl)amino)ethyl)phenyl)-, (2r,3r)-2,3-dihydroxybutanedioate (1:1) (salt)
11. N-[2-hydroxy-5-[(1r)-1-hydroxy-2-[[(1r)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]-formamide (2r,3r)-2,3-dihydroxybutanedioate (1:1) (salt)
12. Brovana Inhalation Solution
13. Unii-5p8vj2i235
14. Brovana (tn)
15. Arformotero Ltartrate
16. Dtxsid80173903
17. Arformoterol Tartrate [vandf]
18. S5217
19. Arformoterol Tartrate [mart.]
20. Akos005145740
21. Arformoterol Tartrate [who-dd]
22. Ccg-269651
23. Arformoterol Tartrate, >=98% (hplc)
24. Ba171831
25. Bs-42158
26. Arformoterol Tartrate [orange Book]
27. Formoterol R,r-form L-tartrate [mi]
28. D02981
29. 815f492
30. Q-101035
31. Q27262686
32. (2r,3r)-2,3-dihydroxybutanedioic Acid;n-[2-hydroxy-5-[(1r)-1-hydroxy-2-[[(2r)-1-(4-methoxyphenyl)propan-2-yl]amino]ethyl]phenyl]formamide
33. (r,r)-formoterol Tartrate; N-(2-hydroxy-5-((1r)-1-hydroxy-2-(((1r)-2-(4-methoxyphenyl)-1-methylethyl)amino)ethyl)phenyl)formamide (2r,3r)-2,3-dihydroxybutanedioate (1:1)
34. [(2r)-2-(3-formamido-4-hydroxyphenyl)-2-hydroxyethyl]-[(2r)-1-(4-methoxyphenyl)propan-2-yl]azanium;(2r,3r)-2,3,4-trihydroxy-4-oxobutanoate
35. N-[2-hydroxy-5-[(1r)-1-hydroxy-2-[[(1r)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formamide (2r,3r)-2,3-dihydroxybutane Dioate
36. N-[2-hydroxy-5-[(1r)-1-hydroxy-2-[[(1r)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formamide (2r,3r)-2,3-dihydroxybutanedioate
37. N-[2-hydroxy-5-[(1r)-1-hydroxy-2-[[(1r)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formamide L-tartrate
| Molecular Weight | 494.5 g/mol |
|---|---|
| Molecular Formula | C23H30N2O10 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 11 |
| Exact Mass | 494.19004516 g/mol |
| Monoisotopic Mass | 494.19004516 g/mol |
| Topological Polar Surface Area | 206 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 521 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Brovana |
| PubMed Health | Arformoterol (By breathing) |
| Drug Classes | Bronchodilator |
| Active Ingredient | Arformoterol tartrate |
| Dosage Form | Solution |
| Route | Inhalation |
| Strength | eq 0.015mg base/2ml |
| Market Status | Prescription |
| Company | Sunovion |
| 2 of 2 | |
|---|---|
| Drug Name | Brovana |
| PubMed Health | Arformoterol (By breathing) |
| Drug Classes | Bronchodilator |
| Active Ingredient | Arformoterol tartrate |
| Dosage Form | Solution |
| Route | Inhalation |
| Strength | eq 0.015mg base/2ml |
| Market Status | Prescription |
| Company | Sunovion |


