



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
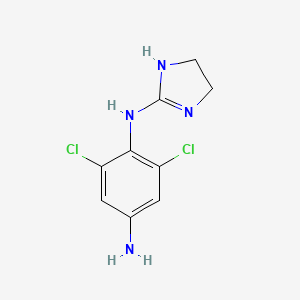

1. 2-(4-amino-2,6-dichloro)phenyliminoimidazolidine
2. 4-aminoclonidine
3. Alo 2145
4. Alo-2145
5. Apraclonidine Hydrochloride
6. Iopidine
7. Iopimax
8. P-aminoclonidine
9. Para-aminoclonidine
1. 66711-21-5
2. 4-aminoclonidine
3. Aplonidine
4. Apraclonidina
5. Apraclonidinum
6. P-aminoclonidine
7. Apraclonidinum [inn-latin]
8. Apraclonidina [inn-spanish]
9. 2,6-dichloro-n1-(4,5-dihydro-1h-imidazol-2-yl)benzene-1,4-diamine
10. 2,6-dichloro-1-n-(4,5-dihydro-1h-imidazol-2-yl)benzene-1,4-diamine
11. Apraclonidine (inn)
12. Iopidine (tn)
13. Chembl647
14. 843cen85di
15. Chebi:2788
16. Apraclonidine [inn]
17. Apraclonidine [inn:ban]
18. Apraclonidine; Lopidine; Nc 14; P-aminoclonidine
19. Unii-843cen85di
20. P-amino Clonidine
21. Aminoclonidine, P
22. Starbld0003083
23. Lopac-a-0779
24. Apraclonidine [mi]
25. Lopac0_000033
26. Schembl34127
27. Apraclonidine [vandf]
28. Apraclonidine [who-dd]
29. Gtpl7117
30. Dtxsid1048415
31. Bdbm81926
32. Zinc20231
33. Bdbm50021812
34. Nsc_51763
35. Pdsp1_000790
36. Pdsp2_000778
37. 1,4-benzenediamine, 2,6-dichloro-n'-(4,5-dihydro-1h-imidazol-2-yl)-
38. 2,6-dichloro-n(1)-(4,5-dihydro-1h-imidazol-2-yl)benzene-1,4-diamine
39. Akos025401361
40. Ccg-204129
41. Db00964
42. Sdccgsbi-0050022.p002
43. Smp1_000016
44. Ncgc00015033-01
45. Ncgc00015033-02
46. Ncgc00015033-03
47. Ncgc00015033-04
48. Ncgc00162050-01
49. Ac-12697
50. Hy-12720
51. Cas_66711-21-5
52. Db-054956
53. Cs-0012296
54. Ft-0602879
55. C07668
56. D07461
57. 2-(4-amino-2,6-dichlorophenylimino)imidazolidine
58. 711a215
59. A918277
60. L000686
61. L013394
62. 2-(4-amino-2,6-dichlorophenylamino)-2-imidazoline
63. 2-(4-amino-2,6-dichlorophenylimino) Imidazolidine
64. Q4781812
65. 2,6-dichloro-n-imidazolidin-2-ylidene-benzene-1,4-diamine
66. 1,4-benzenediamine, 2,6-dichloro-n1-(4,5-dihydro-1h-imidazol-2-yl)-
67. 2,6-dichloro-n1-(imidazolidin-2-ylidene)benzene-1,4-diamine
68. P-aminoclonidine2,6-dichloro-n-imidazolidin-2-ylidene-benzene-1,4-diamine
69. 2,6-dichloro-n-imidazolidin-2-ylidene-benzene-1,4-diamine (p-aminoclonidine)
70. 2,6-dichloro-n-imidazolidin-2-ylidene-benzene-1,4-diamine(p-aminoclonidine)
| Molecular Weight | 245.11 g/mol |
|---|---|
| Molecular Formula | C9H10Cl2N4 |
| XLogP3 | 1.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 244.0282517 g/mol |
| Monoisotopic Mass | 244.0282517 g/mol |
| Topological Polar Surface Area | 62.4 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 247 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Iopidine |
| PubMed Health | Apraclonidine (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | IOPIDINE Ophthalmic Solution contains apraclonidine hydrochloride, an alpha adrenergic agonist, in a sterile isotonic solution for topical application to the eye. Apraclonidine hydrochloride is a white to off-white powder and is highly soluble in w... |
| Active Ingredient | Apraclonidine hydrochloride |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | eq 1% base; eq 0.5% base |
| Market Status | Prescription |
| Company | Alcon |
| 2 of 2 | |
|---|---|
| Drug Name | Iopidine |
| PubMed Health | Apraclonidine (Into the eye) |
| Drug Classes | Antiglaucoma |
| Drug Label | IOPIDINE Ophthalmic Solution contains apraclonidine hydrochloride, an alpha adrenergic agonist, in a sterile isotonic solution for topical application to the eye. Apraclonidine hydrochloride is a white to off-white powder and is highly soluble in w... |
| Active Ingredient | Apraclonidine hydrochloride |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | eq 1% base; eq 0.5% base |
| Market Status | Prescription |
| Company | Alcon |
For prevention or reduction of intraoperative and postoperative increases in intraocular pressure (IOP) before and after ocular laser surgery when used prophylactically. Also used as a short-term adjunctive therapy in patients with open-angle glaucoma who are on maximally tolerated medical therapy requiring additional IOP reduction.
FDA Label
Apraclonidine significantly lowers intraocular pressure with minimal effects on cardiovascular and pulmonary parameters. It lowers intraocular pressure by reducing aqueous humor production and increasing uveoscleral outflow.
Adrenergic alpha-2 Receptor Agonists
Compounds that bind to and activate ADRENERGIC ALPHA-2 RECEPTORS. (See all compounds classified as Adrenergic alpha-2 Receptor Agonists.)
S01EA03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
S - Sensory organs
S01 - Ophthalmologicals
S01E - Antiglaucoma preparations and miotics
S01EA - Sympathomimetics in glaucoma therapy
S01EA03 - Apraclonidine
Absorption
Topical use of apraclonidine ophthalmic solution leads to systemic absorption. Studies of apraclonidine (0.5% ophthalmic solution) dosed one drop three times a day in both eyes for 10 days in normal volunteers yielded mean peak and trough concentrations of 0.9 ng/mL and 0.5 ng/mL, respectively.
8 hours
Apraclonidine is a relatively selective alpha2 adrenergic receptor agonist that stimulates alpha1 receptors to a lesser extent. It has a peak ocular hypotensive effect occurring at two hours post-dosing. The exact mechanism of action is unknown, but fluorophotometric studies in animals and humans suggest that Apraclonidine has a dual mechanism of action by reducing aqueous humor production through the constriction of afferent ciliary process vessels, and increasing uveoscleral outflow.


