


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
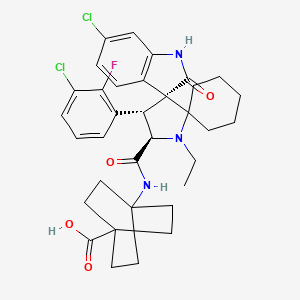

1. Alrizomadlin
2. Aa-115
3. 1818393-16-6
4. 15qau0si9j
5. Apg-115
6. Apg115
7. Apg 115 [who-dd]
8. Chembl4091801
9. 4-((3'r,4's,5'r)-6''-chloro-4'-(3-chloro-2-fluorophenyl)-1'-ethyl-2''-oxodispiro[cyclohexane-1,2'-pyrrolidine-3',3''-indoline]-5'-carboxamido)bicyclo[2.2.2]octane-1-carboxylic Acid
10. (3'r,4's,5'r)-4-((6-chloro-2-oxo-1,2-dihydro-spiro(indole-3,3'-pyrrolidin)e-4'-(3-chloro-2-fluoro -phenyl)-1'-ethyl-spiro(cyclohexane-1,2'-pyrrolidine)-5'-carbonyl)-amino)-bicyclo(2.2.2)octane-1-carboxylic Acid
11. Alrizomadlin [inn]
12. Unii-15qau0si9j
13. Schembl17189805
14. Bdbm50237739
15. Mfcd32197175
16. Nsc831270
17. Nsc-831270
18. Hy-101518
19. Cs-0021621
| Molecular Weight | 642.6 g/mol |
|---|---|
| Molecular Formula | C34H38Cl2FN3O4 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 641.2223403 g/mol |
| Monoisotopic Mass | 641.2223403 g/mol |
| Topological Polar Surface Area | 98.7 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 1160 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


