
Synopsis
Synopsis
0
USDMF
0
JDMF
0
KDMF
0
VMF
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
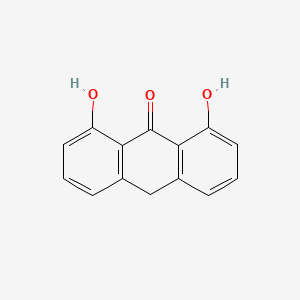

1. 1,8 Dihydroxy 9 Anthrone
2. 1,8,9-anthracenetriol
3. 1,8-dihydroxy-9(10h)-anthracenone
4. 1,8-dihydroxy-9-anthrone
5. Anthraforte
6. Anthranol
7. Cignolin
8. Cygnoline
9. Dihydroxyanthranol
10. Dithranol
11. Dithrocream
12. Ditranol Fna
13. Fna, Ditranol
14. Lasan
15. Micanol
16. Psoradrate
17. Psoricrme
1. Dithranol
2. 1143-38-0
3. 1,8-dihydroxyanthrone
4. Cignolin
5. 1,8-dihydroxy-9-anthrone
6. 1,8-dihydroxyanthracen-9(10h)-one
7. Chrysodermol
8. Cigthranol
9. 9(10h)-anthracenone, 1,8-dihydroxy-
10. Batridol
11. Psoriacid-stift
12. Lasan
13. Anthra-derm
14. 1,8-dihydroxy-10h-anthracen-9-one
15. Drithoscalp
16. Derobin
17. Drithocreme
18. Batidrol
19. Psodadrate
20. Psoriacide
21. Anthrone, 1,8-dihydroxy-
22. 1,8-dihydroxy-9(10h)-anthracenone
23. Nsc 43970
24. Anthraderm
25. Anthraline
26. Nsc 629313
27. Anthralin [usp]
28. Dithranol [inn]
29. Anthralin (dithranol)
30. Chebi:37510
31. Nsc-43970
32. U8cjk0jh5m
33. Nsc-629313
34. Chembl46469
35. Anthralin (usp)
36. Dithranol (inn)
37. Nsc629313
38. Ncgc00091330-01
39. Dsstox_cid_4538
40. 1,8-dihydroxy-9(10h)-anthracenone;anthralin
41. Dsstox_rid_77448
42. Dsstox_gsid_24538
43. Dithranolum
44. Dithranolum [inn-latin]
45. Zithranol-rr
46. Ccris 628
47. Cas-1143-38-0
48. Sr-05000002011
49. Einecs 214-538-0
50. Unii-u8cjk0jh5m
51. Mfcd00053409
52. Brn 2054360
53. Cignoline
54. Ccris 5592
55. Prestwick_528
56. Anthralin, Dithranol
57. Dithranol ,(s)
58. Nsc 403736
59. Brn 1976792
60. Anthrone,8-dihydroxy-
61. Spectrum_000056
62. Anthraderm (tn)
63. Anthralin [mi]
64. Dithranol [iarc]
65. Spectrum2_000111
66. Spectrum3_000304
67. Spectrum4_000151
68. Spectrum5_000820
69. Anthralin [vandf]
70. Dithranol [mart.]
71. Epitope Id:114081
72. 1,8-dihydroxy-9-anthrane
73. Anthralin [usp-rs]
74. Cid_2202
75. Dithranol [who-dd]
76. Dithranol [who-ip]
77. Schembl3197
78. Bspbio_001868
79. Kbiogr_000622
80. Kbioss_000436
81. 4-06-00-07602 (beilstein Handbook Reference)
82. Mls001332632
83. Mls002415712
84. 1,8,9-trihydroxyanthracene;
85. Divk1c_000021
86. Spectrum1500127
87. Spbio_000122
88. Zinc1322
89. Dithranol [ep Impurity]
90. Dithranol [ep Monograph]
91. Dithranol, >=90% (hplc)
92. Dtxsid7024538
93. Hms500b03
94. Kbio1_000021
95. Kbio2_000436
96. Kbio2_003004
97. Kbio2_005572
98. Kbio3_001368
99. Nuzwlkwwnnjhpt-uhfffaoysa-
100. Anthralin [usp Monograph]
101. Ninds_000021
102. Hms1920e07
103. Hms2091k07
104. Hms2271b09
105. Hms3715h19
106. Pharmakon1600-01500127
107. Hy-b0738
108. Nsc43970
109. 9(10h)-anthracenone,8-dihydroxy-
110. Tox21_111115
111. Tox21_201851
112. Tox21_300290
113. Bdbm50041802
114. Ccg-38920
115. Nsc755873
116. S4590
117. 1,8-dihydroxy-9-(10h)anthracenone
118. Akos015914122
119. Tox21_111115_1
120. Db11157
121. Ks-5183
122. Nsc-755873
123. Idi1_000021
124. 1,8-dihydroxy-9(10h)-anthracenone #
125. Ncgc00091330-02
126. Ncgc00091330-03
127. Ncgc00091330-04
128. Ncgc00091330-05
129. Ncgc00091330-07
130. Ncgc00253941-01
131. Ncgc00259400-01
132. Ac-14842
133. Nci60_004019
134. Sbi-0051286.p003
135. Db-060606
136. 1,8-dihydroxy-9,10-dihydroanthracen-9-one
137. Ft-0625372
138. C06831
139. D00233
140. D97650
141. Ab00051916_07
142. A803172
143. Q419397
144. Sr-05000002011-1
145. Sr-05000002011-2
146. W-108602
147. Dithranol, European Pharmacopoeia (ep) Reference Standard
148. Anthralin, United States Pharmacopeia (usp) Reference Standard
149. Anthralin (dithranol), Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 226.23 g/mol |
|---|---|
| Molecular Formula | C14H10O3 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 226.062994177 g/mol |
| Monoisotopic Mass | 226.062994177 g/mol |
| Topological Polar Surface Area | 57.5 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 287 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Stable plaque psoriasis of the skin and scalp. It is also used topically in the management of psoriasis, dermatoses, and alopecia areata. Anthralin is also used in biomedical research due to its effect on EGFR autophosphorylation.
Anthralin is a natural anthraquinone derivative, anti-psoriatic and anti-inflammatory agent. It controls skin growth by reducing the synthesis of DNA and the mitotic activity in the hyperplastic epidermis, normalizing the rate of cell proliferation and keratinization.
Dermatologic Agents
Drugs used to treat or prevent skin disorders or for the routine care of skin. (See all compounds classified as Dermatologic Agents.)
D - Dermatologicals
D05 - Antipsoriatics
D05A - Antipsoriatics for topical use
D05AC - Antracen derivatives
D05AC01 - Dithranol
Absorption
Anthralin penetrates damaged skin and psoriatic lesions faster and to a greater extent than normal skin, likely due to increased vascularity of psoriatic lesions.
Anthralin is administered topically. Although the extent of systemic absorption after topical application has not been determined, no traces of anthraquinone metabolites were detected in the urine of treated subjects in a limited clinical study of anthralin cream,. Anthralin does not inhibit hepatic microsomal enzyme activity.
Anthralin inhibits the proliferation of keratinocytes (epidermal skin cells), prevents the action of T-cells, and promotes cell differentiation, likely through mitochondrial dysfunction. In addition, the production of free radicals may contribute to its anti-psoriatic effect. In vitro studies demonstrate that anthralin prolongs the prophase component of mitosis for keratinocytes and leukocytes. Prophase is the first step of mitosis, the process separating the duplicated genetic material carried in the nucleus of a parent cell into two identical daughter cells. In vivo studies demonstrate that anthralin blocks DNA synthesis and can increase the release of reactive oxygen species. Anthralin is believed to normalize the rate of epidermal cell proliferation and keratinization by reducing the mitotic activity of the epidermal hyperplasia in psoriasis. Anti-proliferative and anti-inflammatory effects of anthralin have been demonstrated on both psoriatic and healthy skin. The anti-proliferative effects of anthralin are thought to be due to a combination of inhibition of DNA synthesis and its strong reducing properties. The effectiveness of anthralin as an anti-psoriatic agent is partly owed to its ability to promote lipid peroxidation and reduce the concentration of endothelial adhesion molecules, which are found to be elevated in psoriatic patients,. Recent studies suggest that its ability to prevent T-lymphocyte activation and normalize keratinocyte differentiation may occur by a direct effect on mitochondria.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
92
PharmaCompass offers a list of Dithranol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Dithranol manufacturer or Dithranol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Dithranol manufacturer or Dithranol supplier.
PharmaCompass also assists you with knowing the Dithranol API Price utilized in the formulation of products. Dithranol API Price is not always fixed or binding as the Dithranol Price is obtained through a variety of data sources. The Dithranol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Anthralin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Anthralin, including repackagers and relabelers. The FDA regulates Anthralin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Anthralin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Anthralin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Anthralin supplier is an individual or a company that provides Anthralin active pharmaceutical ingredient (API) or Anthralin finished formulations upon request. The Anthralin suppliers may include Anthralin API manufacturers, exporters, distributors and traders.
click here to find a list of Anthralin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Anthralin CEP of the European Pharmacopoeia monograph is often referred to as a Anthralin Certificate of Suitability (COS). The purpose of a Anthralin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Anthralin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Anthralin to their clients by showing that a Anthralin CEP has been issued for it. The manufacturer submits a Anthralin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Anthralin CEP holder for the record. Additionally, the data presented in the Anthralin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Anthralin DMF.
A Anthralin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Anthralin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Anthralin suppliers with CEP (COS) on PharmaCompass.
A Anthralin written confirmation (Anthralin WC) is an official document issued by a regulatory agency to a Anthralin manufacturer, verifying that the manufacturing facility of a Anthralin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Anthralin APIs or Anthralin finished pharmaceutical products to another nation, regulatory agencies frequently require a Anthralin WC (written confirmation) as part of the regulatory process.
click here to find a list of Anthralin suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Anthralin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Anthralin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Anthralin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Anthralin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Anthralin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Anthralin suppliers with NDC on PharmaCompass.
Anthralin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Anthralin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Anthralin GMP manufacturer or Anthralin GMP API supplier for your needs.
A Anthralin CoA (Certificate of Analysis) is a formal document that attests to Anthralin's compliance with Anthralin specifications and serves as a tool for batch-level quality control.
Anthralin CoA mostly includes findings from lab analyses of a specific batch. For each Anthralin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Anthralin may be tested according to a variety of international standards, such as European Pharmacopoeia (Anthralin EP), Anthralin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Anthralin USP).

